In this article, we will address the n2 lewis structure and discuss other structural aspects of the intended molecule, such as hybridization, formal charges, shape, and uses.
Nitrogen is a diatomic colorless gas that occupies 78% volume of the earth’s atmosphere having a molecular weight of 28 g/mol. Nitrogen exists as N2 ( Dinitrogen), having three bonds between two nitrogen atoms and ranked as the third most electronegative non-metal after fluorine and oxygen.
How to draw n2 lewis structure?
Before writing lewis structure of any molecule, we need to keep above mention rules in mind.
- Number of valence electrons
- Based on electronegativity, decide which atom will occupy the central position.
- It is an unsaid rule in the chemical world that every atom is a sigma donor first; only then can it contribute further. Hence always establish a single bond between the given atom and then go for a double or triple bond.
- After arranging the shared electrons between the atoms, always check whether the goal of stable configuration is attained or not. Generally, in the resultant molecule, every atom has 8 electrons in its outer shell after sharing the electrons, indicating stability.
By keeping all the rules mentioned above, let’s draw the N2 lewis structure step by step;
- Nitrogen belongs to the 2nd period and 15th family in the periodic table and has an electronic configuration of [He] 2s22p3. It contains five valence electrons.
No valence electrons in case of N ( Z=7) = 5
- since there is the involvement of the same atoms and hence no need to consider electronegativity parameters.
- The last step involve the arrangement of sharing electrons such that after both the atoms must have stable configuration after sharing. Now, lets draw the N2 lewis structure:

N2 lewis structure
N2 lewis structure lone pairs
Lone pairs are those electrons that do not participate in bonding during bond formation or also known as non-bonding electrons. With the help of the lewis structure, one can easily predict how many lone pairs a particular atom can have after bonding. Likewise, the N2 lewis structure indicated the presence of two lone pairs ( one for each nitrogen atom).
N2 lewis structure octet rule
N2 is the first member of the 15th group ie. 2nd group element, having a total of 5 electrons in the outermost orbitals. After the triple bond formations as per the N2 lewis structure, each nitrogen atom has 8 electrons in its orbitals, indicating a stable electronic configuration. According to the octet rule, the main purpose of bonding is to become more stable.
Hence, after overlapping the atoms, they must have 8 electrons in their shells to attain a stable configuration. Since, in the N2 lewis structure, each atom has fulfilled the condition of 8 electrons. Therefore, it obeys the octet rule.
The another aspect of lewis structure is that , one can easily predict by looking at N2 lewis structure that how many electrons were contributed by the each nitrogen. Each atom has 5 electrons individually and each of them contributing 3 electrons to form a stable configuration.
N2 valence electrons
Another aspect of the lewis structure is that one can easily predict by looking at the N2 lewis structure how many electrons were contributed by each nitrogen. Each atom has 5 electrons individually, and each of them contributes 3 electrons to form a stable configuration.
N2 lewis structure formal charge
The formal charge indicates the total charge carried by the molecule and can be calculated for any molecule by using the above formula:
Formal charges = [valence electrons – unbonded electrons – ½ bonded electrons]
N (Z= 7) = [He] 2s22p3 ie each no of valence electrons = 5
Unbonded electron count = 2
Bonded electrons = 6
F.C = 5 – 2 – 6/2 = 0
Hence formal charge on the dinitrogen is zero
N2 hybridization
Lets see how N2 is formed ?
The above diagram describing all the steps for the formation of N2

As shown in the last step, only one S and One p orbitals are involved in overlapping and Hence N2 has sp hybridization while other unhybridized p orbital can overlap with each other to form pi bonds as we know, there are three bonds are present in the N2 structure.
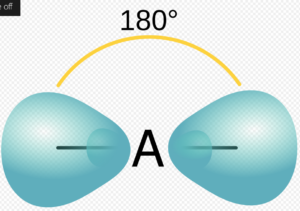
N2 lewis structure angle
As discussed in the hybridization section of N2, it involves SP type hybridization. Therefore, according to VSPER theory it has linear structure with the angle around 180o. The above given diagram illustrates the shape of sp hybridised structure .
N2 uses
Frequently asked questions
What is a nitrogen cycle ?
Due to the abundance of nitrogen in the outer atmosphere, there is a continuous exchange of nitrogen elements between the atmosphere and the biosphere which is known as the Nitrogen cycle.
What is the laboratory preparation of nitrogen?
Generally two ammonium salts such as NH4Cl is treated with aqueous solution to form dinitrogen.
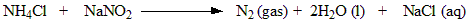
How is N2 isolated from the air ?
Fractional distillation has been used to separate nitrogen from the air as both nitrogen and oxygen are major air components and have different boiling points. Nitrogen has a boiling point of around 77.2 K. In contrast; oxygen has around 90 K . When atmospheric air is heated, nitrogen, due to its comparatively lower boiling point, is distilled out firstly than oxygen. However, dinitrogen always contains some traces of gas impurities.
Give the name of isotopes of N2
The N2 has two stable isotopes, 14N and 15N.
What are the different names of oxides of nitrogen ?
Nitrogen reacts with oxygen in different conditions to give number of binary oxides. Some of them are nitrous oxide (N2O), nitric oxide (NO), nitrogen dioxide (NO2), dinitrogen trioxide (N2O3) and dinitrogen pentoxide (N2O5).
What do you mean by catenation?
Catenation refers to the property of an element that can be capable of forming a bond itself. For instance, nitrogen forms a bond with nitrogen in N2. This property is called catenation.
What are the common oxidation states exhibited by nitrogen?
Nitrogen can exhibit both negative as well as positive oxidation states as in nitrides likeMgN3 N2 showed a -3 oxidation state and +3 oxidation state in NCl3. The highest oxidation state shown by nitrogen is +5.
How to obtain pure nitrogen gas?
Generally, nitrogen gas obtained by fractional distillation is impure and also contains traces of oxygen also. In order to obtain pure nitrogen gas, sodium azide (NaN3) is subjected to thermal decomposition at high temperatures.
Why is N2 unreactive at room temperature?
This is because of the presence of a triple bond which makes nitrogen an inert gas as it has a high bond dissociation energy of around 941.4 KJ/mol.
Why nitrogen do not participate in respiration and other biological processes like O2 does?
Nitrogen is inert and does not react. Therefore, it does not participate. Moreover, because of its unreactive nature, it does not support processes like combustion.
How does nitrogen react with litmus paper?
Nitrogen is neutral in nature and hence does not react with litmus paper.
What is nitrogen fixation?
Atmospheric nitrogen can not directly used by plants, it needs to break into more useful forms like ammonia and the process by which it convert into more useful compounds is called nitrogen fixation.
What do you understand by artificial nitrogen fixation?
When the process of nitrogen fixation is carried out under an artificial environment by setting up suitable chemical conditions, this phenomenon is called artificial nitrogen fixation. The best example of this method is Haber’s process.
Why can nitrogen not form pentahalides?
Nitrogen can show an oxidation state of +5, but still, it cannot form pentahalides because of the absence of d orbitals in its outmost shell.
How does nitrogen react with metals?
Nitrogen combines with the metal to form nitrides, as shown below:
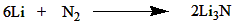
What is the special property exhibited by the nitrogen oxides?
In the 15thfamily, only nitrogen is the only one that can form pπ-pπ multiple bonds, while other members of the family can not establish such bondings in their oxide structures.
What happens when N2 reacts with molecular oxygen?
When nitrogen reacts with O2 it leads to the formation of nitric oxide (NO) in the presence of a high temperature of around 2000K.

Also Read:
- Hocn lewis structure
- Xebr2 lewis structure
- Hio3 lewis structure
- Nacl lewis structure
- Acetone lewis structure
- Bro lewis structure
- Xeo2f2 lewis structure
- Nh2 lewis structure
- Gah3 lewis structure
- Hgbr2 lewis structure

Hello…Myself is Pomila Sharma. I have done my master’s in Chemistry with a specialization in synthetic organic chemistry. I have published 4 research articles. I am very passionate about the chemistry world. I believe it’s all about chemistry so let’s explore it together.