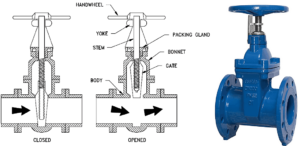
Gate valves are generally made for shut-off, and you find them in almost all plumbing applications from industrial to water supply at home or in gardens.
Needless to say, the same applies for home fluid plumbing as well. To solder a Gate valve following steps are to be followed:
- For smooth functioning and cost-effectiveness of the fluid pipelines, proper soldering of a Gate Valve is mandatory.
- Ensure that the whole piping system is free of any fluid(or water in case of home plumbing) by closing the main valve and draining the residual fluid.
- Before you start the soldering job, ensure the valve is in close condition.
- Measure the length of the Gate valve accurately because that much portion of the pipe has to be cut (generally ½ to ¾ inch depending upon the size of the valve and piping)
- Next, using a pipe cleaner, clean the main pipe as well as the Gate valve. The ends of the pipe must be free of dirt. Cleaning the inside of the pipes is as important.
- Using a flux brush apply some paste flux on both the ends of the main pipe and along with the Gate valve.
- Now insert the cut ends of the main pipe inside the Gate valve properly. Some resistance should be expected.
- For the final step, get the solder wires. Using a propane torch heat both the ends to be soldered. Keep the bent end of solder wire near the adjoined area so that it melts around it properly. Same process is applied for the other end also.
- Allow to cool down the soldered ends and remove the excess amount with the help of a rag.
- The Gate valve is now fixed in the piping system.
Do you Solder a Gate Valve open or closed
Generally, each manufacturer has a recommendation for open or closed state of the Gate valve at the time of soldering. Therefore, it is beneficial to go through the manufacturer’s guidelines before starting the work
As per the experts’ opinion Gate valve should remain in a closed state at the time of soldering. Gate valves are most widely used in industrial sectors for starting or stopping a flow. Gate Valves are not suitable for regulating service.
Before installation of a Gate valve, detailed verification of operating fluid, environment, pressure, and temperature are necessary. The installer should ensure the limit of pressure and temperature that may be sustained by the Gate valve.
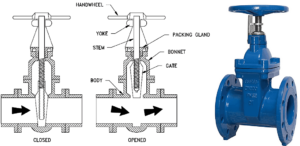
Image credit: Gate valves https://hardhatengineer.com/gate-valve-types-parts/
How do you Solder a copper gate valve?
The soldering flux paste used for copper soldering is Superior No.135 (rosin/petrolatum). This flux provides protection to the soldering area and is ideal for copper soldering.
The basic steps of soldering copper gate valve are as follows:
- Collect all the necessary tools at one place like a propane torch for heating. Arrange tinning flux or paste flux and lead-free solder.
- Cut the pipe with a tube cutter.
- By inserting and twisting a reaming attachment, the inside burrs at the cut ends of the pipe can be removed.
- Clean dirt and corrosion from the outside pipe surface with an emery cloth.
- Inside portion of the pipe should also be cleaned using a wire brush.
- Brush an even layer of flux over the pipe surface and inside the valve, the same way as you apply butter on a toast.
- Heat the joint with the help of a propane torch evenly and allow itthe solder to meltso that it flows into the joint and seal it. A full joint on all sides should be obtained.
How do you Solder a brass gate valve?
The main difference between copper and brass is that brass requires much more heat for the solder to work efficiently.
As an alloy of copper and zinc, Brass is companionable with copper. Solder adheres to copper as well as brass properly, and fittings are generally molded with slip joints and can be easily soldered to the pipes.
More often than not, you would encounter a brass valve while looking for a valve to solder in a copper pipe. The amount of heat required to melt the flux inside a brass valve is around 5-6times that of a copper valve. Hence, in order to ensure that the flux has properly set in between the piping and the valve, sufficient time must be provided for heating.
What position should a gate valve be is to be soldered?
Gate valves are suitable for all types of applications, both above-ground and underground installations.
Most convenient position for all models of Gate valves (inclined, horizontal, and vertical) with the flow in both directions is installed horizontally with the hand wheel pointing in the upward direction.
The position should be selected in such a way so that it can be easily accessible during operation, inspection, and maintenance. A horizontally placed Gate valve is always easy to operate and also easily accessible for maintenance. However, there are some industrial applications where installing a Gate valve vertically downward is preferred. This is to ensure that the valve remains in an open position, even if the lock-nut holding the gate to the spindle has broken and the gate has fallen off.
How to Solder a ball valve?
Soldering a ball valve is a very familiar and frequently used project in plumbing. The soldering technique of ball valves is not a complex process and can be performed without any professional guidance.
To perform correctly, one should have proper knowledge of the whole process. The step by step process for soldering a ball valve is as follows.
- Assemble all the necessary tools like tinning flux, lead-free solder, a ball valve, pipe cutter, pipe cleaner, heat source, safety gloves, etc
- Cut the pipe with the help of a pipe cutter and clean the two ends thoroughly.
- Apply a layer of flux with the help of a brush on the two pipe ends and inside the ball valve also.
- Place the ball valve in between the cut ends of the pipe,
- According to the manufacture’s guidelines, keep the ball valve in the open or closed position during soldering.
- Apply heat to the pipe and ball valve area. It is important to note that the body of the valve should not come under direct fame as otherwise, the nylon seal inside the valve may be melted.
- Apply the solder on adjoined areas and continue heating until the solder melts and fills up the gap. Let the pipe cool down.
- One of the most commonly asked questions is whether to solder ball valves in an open or closed position.Both open and closed option is suitable for soldering ball valves. But it is advisable to solder ball valves in a closed position to avoid the chance of formation of blowing gas bubbles in the joint.
- In the closed position, air generally exits the pipe. On the contrary, there are certain risks involved in soldering a ball valve in an open position. In the open position, water may be trapped in the seals or pipes, which get convert into steam due to the application of heat during soldering. Steam may blow out the seal causing damage to the valve.

Image Credit: Soldering of Ball valves https://www.pressreader.com/usa/the-family-handyman/20180501/282437054662433
Can I Solder a closed ball valve with water on the other side?
Soldering a closed valve with water on the side is beneficial.
It is possible to solder a ball valve with one side in live condition. The application of heat during soldering should not be too high to melt the seals. Otherwise, it may result from a bleeder port. Wrapping a wet rag is always suggested for better results.
To remove the heat produced during soldering, it is normal practice to wrap a wet cloth to remove the heat away. If water is present on the other side, it is beneficial as it will help to remove the heat generated by the soldering process. Care must be taken so that you don’t heat the side with water as it might lead to vaporization of water leading to the blow-up of the ball valve seal.
Can you Solder near Teflon tape?
Teflon tapes or PTFE (Polytetrafluoroethylene) is a common name in plumbing, most widely used for sealing pipe threads. Breakdown may occur in contact with open flames.
The applicable temperature range of a Teflon tape ranges from -268°C to+260°C. Generally, a Teflon tape can withstand temperatures upto 260°C, rate of decomposition is slow up to 400°C. During Soldering near PTFE tape, this temperature range should be maintained.
What is used in Soldering?
Soldering is a joining process most widely used in manufacturing electronic equipment, joining and sealing pipes in the plumbing trade, and also in the jewelry business.
The list of essential items required for any kind of Soldering process are:
- Solder: The main ingredient of the Soldering technique melts to join different types of metals. Traditionally an alloy of tin and lead (Sn 60% & Pb 40%). Nowadays, due to lead toxicity, most of the solders are lead-free, which is an alloy of tin with other metals like copper and silver.
- Soldering Iron: It is a handheld tool that is the main source of heat to melt the solder. Generally, it has a pencil-like shape and very comfortable and easy-to-handle tool. As per the requirement, it may also be available as larger solder guns.
- Soldering Flux:To achieve a good solder joint, a fresh and proper chemical flux is essential. Flux removes oxides from both the metal surface and solder surface so that molten solder can wet the clean metal surfaces to be joined. Resin, Organic, and Inorganic are the different types of flux used as per the requirement.
Soldering valve to copper pipe
Soldering a valve to the copper pipe can be tricky or an easy task at the same time.
The basic steps and precautions that are to be taken for proper soldering of a valve to a copper pipe are as follows:
- The workmanship of an effective Copper pipe soldering depends largely on sticking to the basics, which are: – right preparation, right tools, and rights methods
- Get all your tools in places like the flux paste, the propane torch, the solder, a piece of sandpaper, the pipe cutter, and of course, the valve and the pipe.
- If the soldering is in a live line, ensure the line is drained properly and the source of inlet water is isolated. Then cut the pipes with the pipe cutter.
- Once the pipes are cut, it is necessary that the surfaces at both ends of the cut pipe are properly rubbed with the abrasive sandpaper so that a shine appears over the surfaces to be soldered.
- The next step is to dry-fit the valve, and it is to be ensured that the valve fits into both sides snugly.
- Apply the flux properly on both the surface as you apply butter to a toast!!! And install the valve.
- Before you start heating, ensure the valve is at least partially open. This is done so that if there is any residual water left in the pipe, it doesn’t pressurize the system and crack the soldered joints.
- Lit up the propane torch to heat the surface to melt the flux within.
- Once the flux has melted, the primary sealing is done. The next step is to bring in the solder.
- Continue heating with the propane torch to melt the solder so that it is assimilated into the gap between the pipe and the fitting, thus providing a leak-proof fitting.
- Once it is ensured that the solder has got into all the cracks and crevices of the fitting, let it cool down before being put into service. This is to ensure that the newly soldered joint does not crack due to thermal shock.
How to Solder brass valve to copper?
It is not a big deal to solder brass valve to copper pipe if we are aware enough about certain facts. The temperature of the pipe should be hot enough at the time of applying the Solder.
It is advisable to perform soldering of brass valves correctly in the first attempt because redo may create the problem. When the metal surface reaches the accurate temperature, the plumbing solder flows into the joint between the pipe surface and valve by capillary action and, after cooling, results in a watertight seal.
To avoid a leaking joint, make sure that the moisture inside the pipe can escape completely when it turns into steam.
The same steps are followed as discussed above for soldering valve to copper pipe.