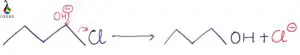
In this article, we will have a closer approach towards the nucleophilic substitution reaction mechanism, i.e., SN1 with appropriate SN1 examples.
What does the one stands for in the SN1 mechanism? Means that it follows first-order, or we can say the reaction rate depends only on the concentration of substrate. We will analyze the factors governing the reaction mechanism with various SN1 examples like carbocation stability, carbon skeleton, the leaving group, solvent, etc.
This reaction occurs in 2 steps:
Step 1: Formation of the carbocation.
Step 2: Reaction of carbocation with the nucleophile
Factors affecting SN1 mechanism :

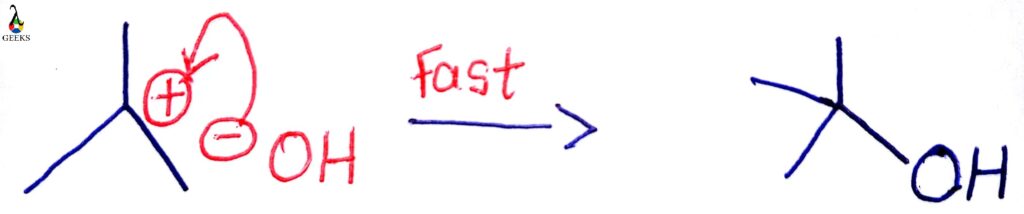
Carbon skeleton structure: compounds forming stable carbocations react by the SN1 mechanism. Also, the substrate, which contains good leaving groups and adjacent substituents like a phenyl group that stabilizes a positive charge mesomerically, are prone to undergo SN1 reaction.
More substituted carbocation, i.e., tertiary carbon, readily forms carbocation, which is very stable. The stability of carbocation formed as an intermediate in the reaction plays a crucial role in determining the efficiency of the SN1 reaction.
Stability order of carbocation:
Tertiary > secondary > primary
Note: Planarity is so essential to the structure of a carbocation that if a tertiary cation cannot become planar, it is not formed.
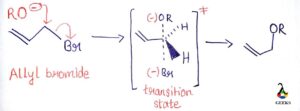
Allylic electrophiles react well by the SN1 mechanism as the allyl cation is relatively stable , important sn1 example.
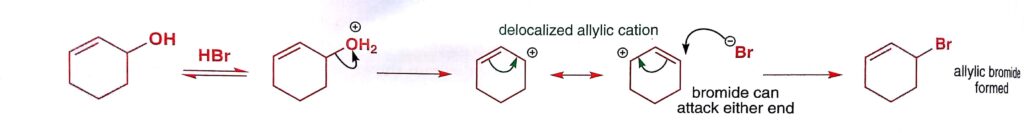
Image Credit: Organic Chemistry by Jonathan Clayden, Nick Greeves, and Stuart Warren.

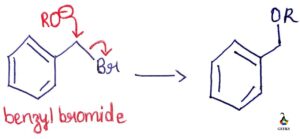
Image Credit: Organic Chemistry by Jonathan Clayden, Nick Greeves, and Stuart Warren.
An exceptional stable cation is formed when the three benzene rings stabilize the same positive charge. The resultant is the triphenylmethyl cation or trityl cation. Trityl chloride is used to form an ether with a primary alcohol group by the SN1 reaction.
Pyridine is used as a solvent for the reaction. Pyridine is not strong enough to remove the proton from the primary alcohol, and there would be no point in using a base strong enough to make RCH2O- as the nucleophile makes no difference to an SN1 reaction. Instead, the TrCl ionizes first to trityl cation, which now captures the primary alcohol, and finally, pyridine can remove the proton from the oxonium ion.
Pyridine does not catalyze the reaction; it stops it from becoming too acidic by removing the HCl formed. Pyridine is also a suitable polar organic solvent for ionic reactions.
Note: The reaction rate depends only on the concentration of substrate alone.
Nucleophile: Since the nucleophile comes into the picture after the rate-determining step, the strength or reactivity of a nucleophile plays no significant role in the SN1 mechanism.
Read more about : Biosynthesis | A ray of hope for the environment and biotechnology
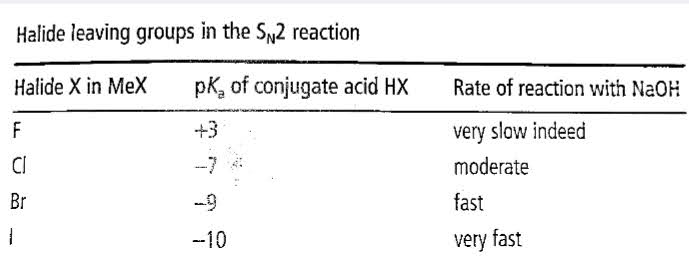
The effect of leaving group:
- In the SN1 mechanism, the C-leaving group breaks and plays a pivotal role in the rate-determining step.
- It is essential to have an excellent leaving group so that the reaction is exothermic (spontaneous).
- The strength of the bond between the leaving group and carbon should be weak.
- It should be stable enough so that it does not have the ability to recombine with carbon.
- A reliable/preferred leaving group is said to be a conjugate base of a strong acid.
Basicity regarding Ions of Halides

Iodine is the most stable base, and F- is said to be the least stable base.
The efficiency of ions of Halides as leaving group :
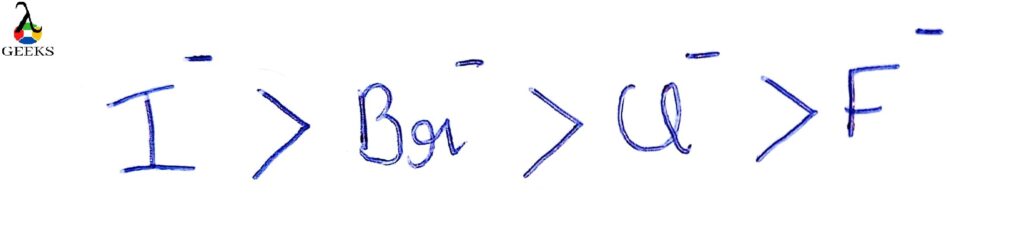
I is said to be the best leaving group and F- not an excellent leaving group.
A group that can appropriately accommodate electrons is considered an excellent leaving group.
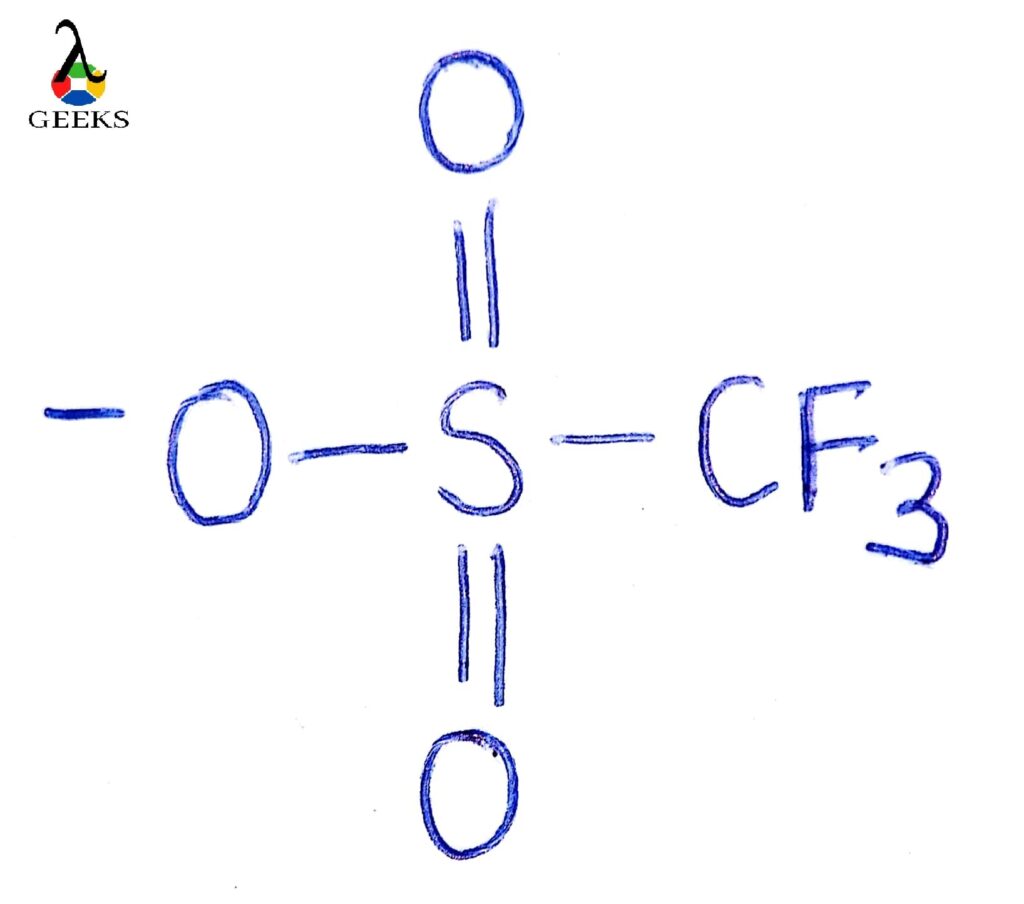
Triflate ion is considered as an outstanding leaving group.
Conversion of bad leaving group to an excellent leaving group
Read more about : Fluorescence Microscopy | Definition | Working | 3 Important Limitations
- By carrying protonation
- Converting ROH into an Ester, i.e., sulfonic Ester group is relatively a good leaving group.
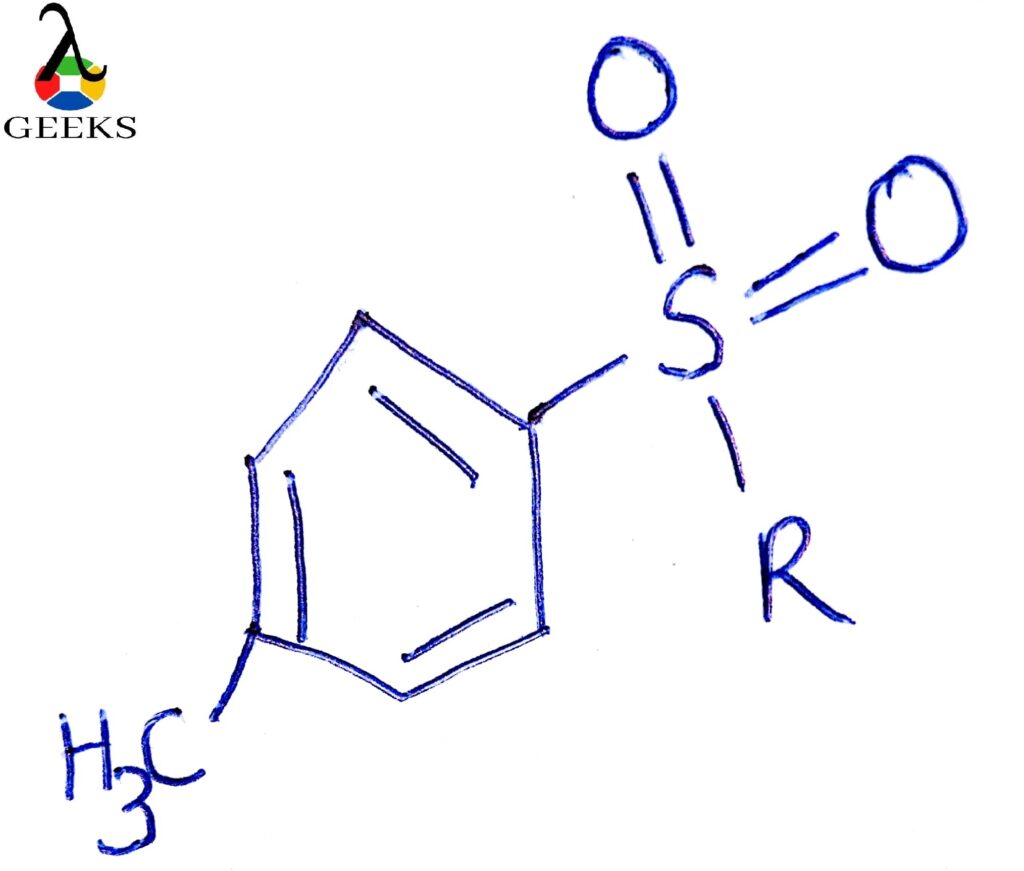
Effect o solvent: It is usually carried out in polar protic solvents because polar solvents stabilize the intermediate carbocation, increasing the rate of reaction. Reduces the effect of the nucleophile, solvates the carbocation and anion, thus imparts stability and hence favors SN1 mechanism.
In Sn1 mechanism polarity of the solvent is proportional to its respective dielectric constant. Ionization occurs faster in solvents with high polarities, such as water or alcohol.

Image credit: https://youtu.be/ep-X4KCvtRk
Example: Consider the rate of solvolysis of 2-Bromo 2-methyl propane is said to be 3 × 104 times faster in 50% aqueous ethanol as compared to in 100% ethanol. This is the charge that is developed in the intermediate ion. As the polarity of the solvent increases, the stability of carbonium ion increases, and therefore rate of SN1 reaction increases. Essential sn1 example.
Read more about : Stereo Microscope | 3 Important parts | Magnification | Illumination
Stereochemistry involved in SN1 mechanism:
- Product is obtained with racemization and varying degree of inversion of configuration.
- Carbocation stability is essential as it is directly proportional to racemization.
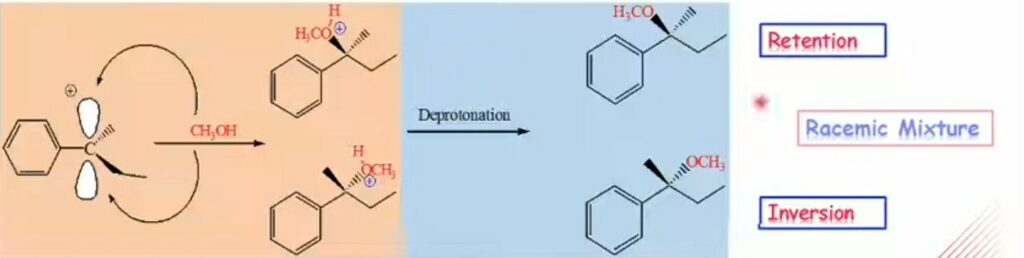
Image Credit: YouTube
Ion pairs in SN1 mechanism:
We know that carbocation carbon atoms consist of sp2 hybridization, so it should be planar and hence achiral. In the reaction of a chiral substrate, the attack can be at either side of the plane, yielding equal amounts of enantiomeric products formation, giving a racemization mixture.
At the time of many SN1 reactions, sometimes there is 6 to 21% inversion, and a sometimes small amount of configuration is retained. These results can be explained by citing the formation of ion pairs.

Image credit: Organic Chemistry mechanism by Ahluwalia
In contact or intimate ion pair, i.e., R+ does not act as a free cation of dissociated species. It is observed that the bond formation between R+ and X- the symmetry is adequately maintained, i.e., the originality of stereochemical configuration is retained by individual ions.
Hence in the SN1 mechanism, X- solvates a cation from the side where it is leaving, and the molecules of solvent around the intimate ion pair solvate only from the opposite side. This gives rise to the situation when carbocation is unsymmetrically solvated. On the attack of nucleophiles, the solvent molecules produce inverted products.
When a nucleophile attacks carbocations of solvent separated ion pair, the carbocations stereochemistry is not maintained tightly. There are chances that the leaving group can block the approach of a nucleophile to that particular side of the carbocation. Therefore formed products will have configuration inverted, giving a mixture of racemization.
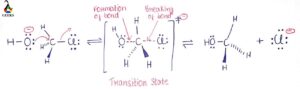
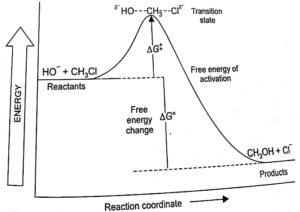
Energy profile diagram of SN1 reaction: sn1 example t-butyl bromide and water

Image credit: Jonathan Clayden, Nick Greeves, and Stuart Warren
The carbocation is shown as an intermediate- a species with a finite (short) lifetime. And because we know that the first step, the carbocation formation, is slow, that must be the step with the higher energy transition state.
The energy of that transition state, which determines the overall rate of the reaction, is closely linked to the stability of the carbocation intermediate, and it is for this reason that the most important factor in determining the efficiency of the SN1 mechanism is the stability or otherwise of any carbocation that might be formed as an intermediate.
FAQs
1.How is the C-X bond polarized in the sn1 mechanism ?
It happens due to the electronegativity of halogens .
2. Why are sn1 reactions ?
It is said unimolecular because the rate of reaction depends only on concentration.
3.Why is it that the SN1 mechanism prefers polar protic solvents?
It prefers polar protic solvents because it stabilizes carbocation intermediate and reduces the activity of nucleophiles, and favors SN1 reaction.
4.What role does concentration play in the SN1 mechanism?
It depends only on substrate concentration and has no effect on the concentration of nucleophiles.
Also Read: