Adenine is a nucleobase that is commonly found in DNA and RNA. It is one of the four nucleobases that make up the genetic code, along with cytosine, guanine, and thymine (in DNA) or uracil (in RNA). Adenine plays a crucial role in the structure and function of nucleic acids, as it forms base pairs with thymine (in DNA) or uracil (in RNA). Additionally, adenine is also involved in various cellular processes, such as energy transfer and signal transduction. Overall, adenine is an essential component of genetic material and plays a vital role in the functioning of living organisms.
Key Takeaways
| Adenine | |
|---|---|
| Structure | Purine base |
| Formula | C5H5N5 |
| Function | Component of DNA and RNA |
| Base pairing | Adenine pairs with thymine (DNA) or uracil (RNA) |
| Cellular processes | Energy transfer, signal transduction |
Understanding Adenine
Adenine is a fundamental component of nucleic acids, which are the building blocks of DNA and RNA. It plays a crucial role in genetic information storage and transfer. Let’s explore some key aspects of adenine to gain a better understanding.
Is Adenine an Amino Acid?
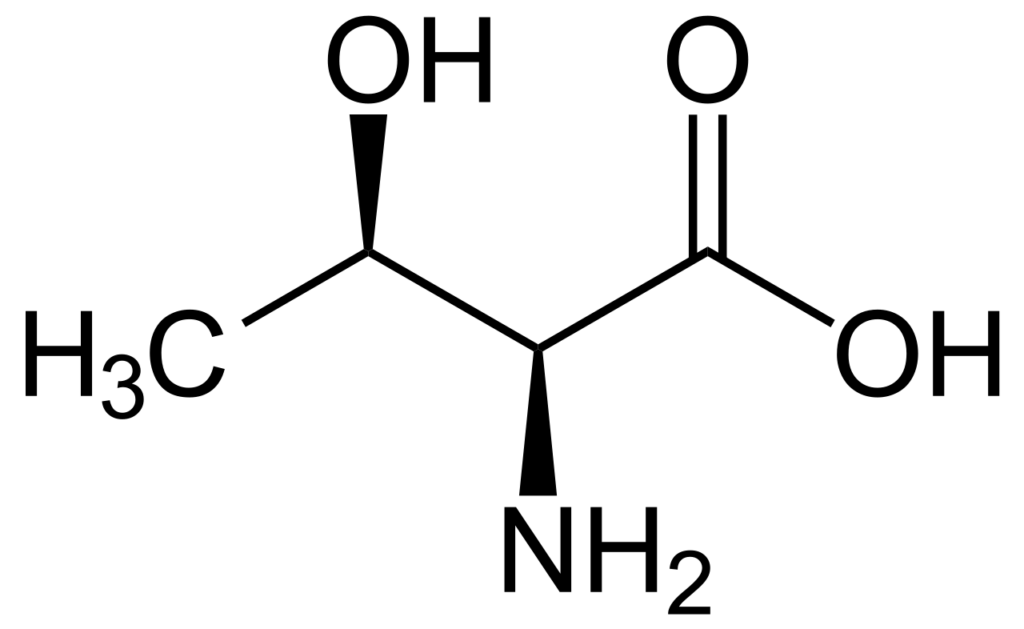
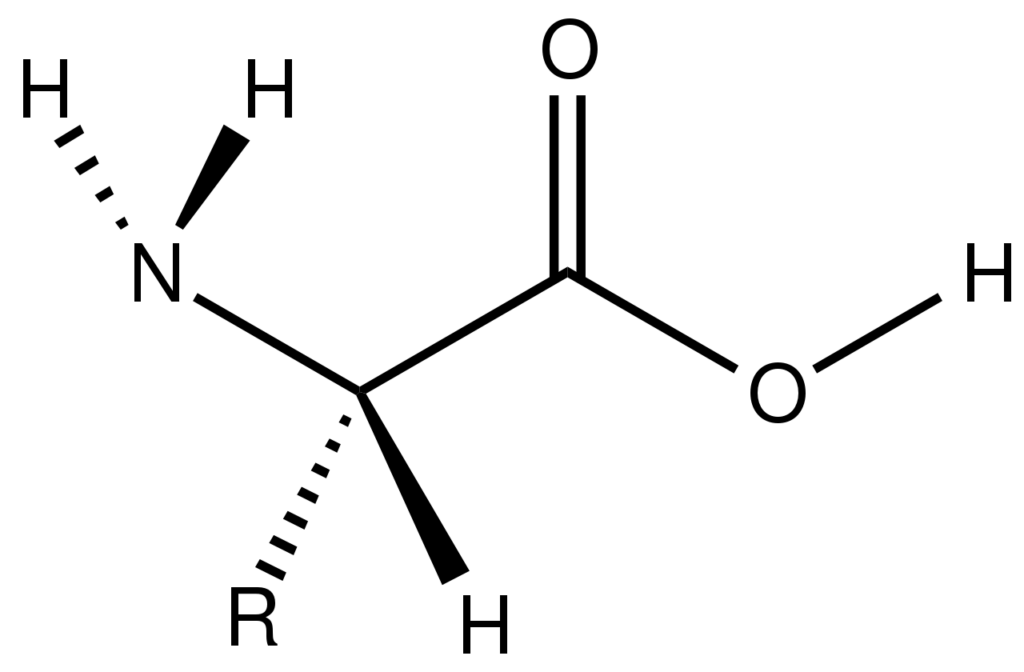

No, adenine is not an amino acid. Amino acids are the building blocks of proteins, whereas adenine is a nucleotide base found in DNA and RNA.
Is Adenine an Element?
Adenine is not an element. It is a nitrogenous base that is part of the larger group of organic compounds known as purines.
Is Adenine an Allele?
No, adenine is not an allele. Alleles are different forms of a gene that can occupy the same position on a chromosome. Adenine, on the other hand, is a specific nucleotide base found in DNA and RNA.
Is Adenine a Purine?
Yes, adenine is classified as a purine. Purines are one of the two types of nitrogenous bases found in nucleic acids, with the other being pyrimidines. Adenine pairs with thymine in DNA and with uracil in RNA through hydrogen bonding.
Is Adenine a Phosphate?
Adenine itself is not a phosphate. However, it can combine with a sugar molecule and one or more phosphate groups to form a nucleotide. Nucleotides are the building blocks of DNA and RNA.
Is Adenine a Sugar?
No, adenine is not a sugar. It is a nitrogenous base. Sugars, such as deoxyribose in DNA and ribose in RNA, combine with adenine and other nucleotide bases to form the backbone of nucleic acids.
Is Adenine an Alkaloid?
Yes, adenine is classified as an alkaloid. Alkaloids are naturally occurring organic compounds that often have pharmacological effects. Adenine is found in various plants and is involved in various biological processes.
Is Adenine an Acid or Base?
Adenine is a base. In the context of nucleic acids, bases are the complementary pairs that form the rungs of the DNA double helix. Adenine specifically pairs with thymine in DNA and with uracil in RNA.
These characteristics of adenine, including its structure, function, and role in DNA and RNA, contribute to the overall understanding of nucleic acids and their importance in genetic information storage and transfer.
Is Adenine an Organic Molecule?
Yes, adenine is an organic molecule. It is a nitrogenous base that is found in both DNA and RNA, two essential molecules for life. Adenine is one of the four bases that make up the genetic code, along with guanine, cytosine, and thymine (in DNA) or uracil (in RNA). It plays a crucial role in the structure and function of these nucleic acids.
Is Adenine a Nucleotide?
To understand whether adenine is a nucleotide, let’s first discuss what a nucleotide is. Nucleotides are the building blocks of nucleic acids, which are the molecules responsible for storing and transmitting genetic information. A nucleotide consists of three components: a nitrogenous base (such as adenine), a sugar molecule, and a phosphate group.
Adenine, as mentioned earlier, is one of the nitrogenous bases found in nucleotides. When adenine combines with a sugar molecule (ribose in RNA or deoxyribose in DNA) and a phosphate group, it forms a nucleotide called adenosine monophosphate (AMP). This nucleotide serves as a precursor for the synthesis of more complex molecules like adenosine triphosphate (ATP), which is the primary energy currency of cells.
In DNA, adenine pairs with thymine through hydrogen bonding, forming a stable base pair. This base pairing is essential for the double helix structure of DNA and its ability to store and transmit genetic information. In RNA, adenine pairs with uracil instead of thymine.
Here are some key properties and characteristics of adenine:
- Adenine is a purine base, meaning it has a double-ring structure.
- Its chemical formula is C5H5N5.
- Adenine is synthesized in the body through various metabolic pathways.
- It is involved in various cellular processes, including energy transfer and signal transduction.
- Adenine is also found in other molecules like adenosine, which is a nucleoside consisting of adenine and a ribose sugar.
In conclusion, adenine is indeed an organic molecule and an essential component of nucleotides in DNA and RNA. Its structure, function, and role in base pairing contribute to the overall stability and genetic information storage of these nucleic acids.
The Structure and Composition of Adenine
Adenine is an essential component of nucleic acids, which are the building blocks of DNA and RNA. It plays a crucial role in the genetic code and is one of the four nucleobases found in DNA. Adenine is a purine base, meaning it has a double-ring structure, and it pairs with thymine in DNA and uracil in RNA.
What Makes Up Adenine?
Adenine is composed of several elements that come together to form its structure. It consists of carbon, hydrogen, nitrogen, and oxygen atoms. The molecular formula of adenine is C5H5N5, indicating its composition of five carbon atoms, five hydrogen atoms, and five nitrogen atoms.
What is Adenine Made Out Of?
Adenine is made up of a complex arrangement of atoms that form its distinct structure. The carbon atoms in adenine are connected to each other in a ring formation, with nitrogen atoms positioned at specific locations within the ring. Hydrogen atoms are attached to the carbon and nitrogen atoms, completing the molecular structure of adenine.
How Big is Adenine?
In terms of size, adenine is relatively small compared to other molecules. It has a molecular weight of approximately 135.13 grams per mole. The compact size of adenine allows it to fit within the DNA double helix, where it forms base pairs with other nucleobases.
What Does Adenine Look Like?
Adenine has a distinct molecular structure that gives it its characteristic appearance. It is a flat molecule with a planar arrangement of atoms. The double-ring structure of adenine consists of a six-membered carbon-nitrogen ring fused with a five-membered carbon-nitrogen ring. This arrangement creates a symmetrical shape with alternating single and double bonds.
In summary, adenine is a vital component of DNA and RNA, playing a crucial role in genetic information storage and transfer. Its structure consists of carbon, hydrogen, nitrogen, and oxygen atoms arranged in a double-ring formation. Adenine’s small size and unique molecular structure allow it to participate in base pairing within the DNA double helix.
The Function and Role of Adenine
Adenine is a vital molecule that plays a crucial role in various biological processes. It is one of the four nitrogenous bases found in DNA and RNA, making it an essential component of genetic material. Adenine is also a key building block of adenosine triphosphate (ATP), the primary energy currency of cells. Let’s explore the importance and functions of adenine in more detail.
Why is Adenine Important?
Adenine holds significant importance due to its involvement in fundamental biological processes. Here are some reasons why adenine is important:
-
Adenine in DNA: Adenine forms base pairs with thymine in DNA, creating a stable double-stranded structure. This base pairing is crucial for DNA replication and transcription, which are essential processes for the transmission of genetic information.
-
Adenine in RNA: Adenine is also present in RNA, where it pairs with uracil. RNA plays a vital role in protein synthesis, and adenine’s presence ensures the accurate transfer of genetic information from DNA to proteins.
-
Adenine Nucleotide: Adenine is a key component of adenosine triphosphate (ATP), a molecule that stores and transfers energy within cells. ATP is involved in various cellular processes, including muscle contraction, active transport, and enzyme reactions.
What is Adenine Used For?
Adenine has several important uses in biological systems. Here are some notable uses of adenine:
-
ATP Synthesis: Adenine is a critical component of ATP, where it forms a bond with ribose sugar and three phosphate groups. This high-energy molecule is synthesized in cells through various metabolic pathways and serves as a universal energy source.
-
Cell Signaling: Adenine derivatives, such as cyclic adenosine monophosphate (cAMP), act as secondary messengers in cell signaling pathways. These molecules transmit signals from the cell surface to the nucleus, regulating various cellular processes.
-
Coenzyme Function: Adenine is a part of coenzymes like nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD). These coenzymes play crucial roles in redox reactions and energy metabolism.
Why is Adenine in ATP?
Adenine is a fundamental component of ATP due to its ability to store and transfer energy. The presence of adenine allows ATP to undergo hydrolysis, releasing energy that can be utilized by cells. This energy release occurs when one of the phosphate groups is cleaved from ATP, converting it into adenosine diphosphate (ADP) and inorganic phosphate (Pi).
Why is Adenine Important in ATP?
Adenine’s importance in ATP lies in its role as a key component of the molecule responsible for cellular energy transfer. ATP provides the necessary energy for various cellular processes, including muscle contraction, active transport, and biosynthesis. Without adenine, ATP would not be able to fulfill its vital role as an energy currency in cells.
Where is Adenine Found?
Adenine is found in various biological molecules and organisms. Here are some notable sources of adenine:
-
DNA and RNA: Adenine is one of the four nitrogenous bases found in DNA and RNA. It is present in the genetic material of all living organisms, from bacteria to humans.
-
Food Sources: Adenine is present in various food sources, including meat, fish, legumes, and grains. These dietary sources contribute to the adenine content in our bodies.
-
Cellular Metabolism: Adenine is synthesized within cells through metabolic pathways. It is produced from precursor molecules and incorporated into nucleotides, coenzymes, and other essential molecules.
In conclusion, adenine plays a crucial role in DNA and RNA structure, ATP synthesis, cell signaling, and energy metabolism. Its presence in biological systems is vital for the proper functioning of cells and the transmission of genetic information.
The Relationship of Adenine with Other Molecules
Adenine is a vital molecule that plays a crucial role in various biological processes. It is one of the four nucleobases found in DNA and RNA, along with guanine, cytosine, and thymine (in DNA) or uracil (in RNA). Let’s explore the relationship of adenine with other molecules in more detail.
Is Adenine and Adenosine the Same?
No, adenine and adenosine are not the same. Adenine is a nucleobase, while adenosine is a nucleoside. Adenosine consists of adenine bonded to a ribose sugar molecule. Adenosine is an important component of RNA and also serves as a building block for the synthesis of ATP (adenosine triphosphate), a molecule that provides energy to cells.
Is Adenine and Guanine Equal?
Adenine and guanine are not equal; they are distinct nucleobases. Adenine has a purine structure, characterized by a double-ring structure, while guanine is also a purine with a similar double-ring structure. Both adenine and guanine are essential components of DNA and RNA, contributing to the genetic code.
When Does Adenine Pair with Uracil?
Adenine pairs with uracil in RNA. In RNA, uracil replaces thymine found in DNA. Adenine forms a complementary base pair with uracil through hydrogen bonding. This pairing is crucial for the proper functioning of RNA molecules in processes such as protein synthesis.
What Does Adenine Pair With?
In DNA, adenine pairs with thymine. This pairing is based on hydrogen bonding, where adenine forms two hydrogen bonds with thymine. Adenine-thymine base pairing is fundamental for the stability and replication of DNA.
Is Adenine and Thymine a Covalent Bond?
No, adenine and thymine do not form a covalent bond. Instead, they form hydrogen bonds between their nitrogenous bases. These hydrogen bonds provide stability to the DNA double helix structure.
Is Adenine and Guanine Purines?
Yes, both adenine and guanine are purines. Purines are nitrogenous bases that have a double-ring structure. Adenine and guanine are essential purine bases found in DNA and RNA.
Is Adenine and Guanine Pyrimidines?
No, adenine and guanine are not pyrimidines. Pyrimidines are nitrogenous bases that have a single-ring structure. The pyrimidines found in DNA and RNA are cytosine, thymine (in DNA), and uracil (in RNA).
Is Adenine and Guanine the Same?
No, adenine and guanine are not the same. They are distinct nucleobases with different structures and properties. Adenine is a purine, while guanine is also a purine. However, they have different chemical compositions and play different roles in DNA and RNA.
In summary, adenine is a crucial molecule that forms the building blocks of DNA and RNA. It pairs with thymine in DNA and uracil in RNA, contributing to the genetic code and various biological processes. Adenine and guanine are both purines but have distinct properties and functions. Understanding the relationship of adenine with other molecules is essential for comprehending the intricate workings of genetics and molecular biology.
Common Misconceptions about Adenine
Adenine is a fundamental component of nucleic acids, such as DNA and RNA, and plays a crucial role in various biological processes. However, there are several misconceptions surrounding adenine that we will address in this section.
Is Adenine a Gene?
No, adenine is not a gene. Adenine is one of the four nucleotide bases found in DNA and RNA, along with guanine, cytosine, and thymine (in DNA) or uracil (in RNA). Genes, on the other hand, are segments of DNA that contain the instructions for building proteins. Adenine is not responsible for encoding specific genetic information but rather serves as a building block for the genetic code.
Is Adenine a Protein?
No, adenine is not a protein. Proteins are large, complex molecules composed of amino acids, whereas adenine is a small molecule known as a purine base. Adenine is involved in the structure and function of nucleic acids, while proteins perform a wide range of functions in the body, including enzymatic activity, cell signaling, and structural support.
Is Adenine a Lipid?
No, adenine is not a lipid. Lipids are a diverse group of molecules that include fats, oils, and waxes. They are characterized by their hydrophobic nature and play essential roles in energy storage, insulation, and cell membrane structure. Adenine, on the other hand, is a nitrogenous base and does not possess the characteristics of a lipid molecule.
Is Adenine a Pentose Sugar?
No, adenine is not a pentose sugar. Pentose sugars, such as ribose and deoxyribose, are the sugar components found in nucleotides, which are the building blocks of nucleic acids. Adenine, as mentioned earlier, is a purine base and does not contain a sugar component. However, adenine does form hydrogen bonds with the sugar component of nucleotides in DNA and RNA, contributing to the stability and structure of the nucleic acid molecules.
Is Adenine a Macromolecule?
No, adenine is not a macromolecule. Macromolecules are large, complex molecules composed of smaller subunits. Adenine, as a single purine base, is not considered a macromolecule. However, when combined with other nucleotide bases, it contributes to the formation of DNA and RNA, which are macromolecules responsible for storing and transmitting genetic information.
In summary, adenine is not a gene, protein, lipid, pentose sugar, or macromolecule. It is a crucial component of nucleic acids, contributing to the structure and function of DNA and RNA. Understanding the role and characteristics of adenine helps to dispel these common misconceptions and provides a clearer picture of its significance in biological processes.
| Misconception | Correct Information |
|---|---|
| Is Adenine a Gene? | Adenine is a nucleotide base, not a gene. |
| Is Adenine a Protein? | Adenine is a purine base, distinct from proteins. |
| Is Adenine a Lipid? | Adenine is not a lipid; it is a nitrogenous base. |
| Is Adenine a Pentose Sugar? | Adenine does not contain a sugar component like pentose sugars. |
| Is Adenine a Macromolecule? | Adenine is not a macromolecule but contributes to the formation of DNA and RNA. |
What is the Purpose of RNA Polymerase in the Synthesis of Adenine?
RNA polymerase is an essential enzyme involved in the synthesis of RNA molecules. In the specific context of adenine synthesis, RNA polymerase plays a crucial role in transcription. There are three types of rna polymerase, one of which recognizes DNA sequences known as promoters and facilitates the synthesis of RNA strands containing adenine bases. This process is fundamental for gene expression and the production of proteins.
Conclusion
In conclusion, adenine is an essential component of DNA and RNA, playing a crucial role in the genetic code of all living organisms. It is one of the four nitrogenous bases found in DNA, along with guanine, cytosine, and thymine. Adenine pairs with thymine in DNA and with uracil in RNA, forming the building blocks of genetic information. Additionally, adenine is involved in various cellular processes, such as energy transfer and signal transduction. Its presence is vital for the proper functioning and replication of genetic material. Overall, adenine is a fundamental molecule that contributes to the complexity and diversity of life on Earth.
Question: Is adenine used in DNA replication and is it considered an amino acid?
Yes, adenine is used in DNA replication. It is one of the four nucleotide bases found in DNA, along with guanine, cytosine, and thymine. Adenine pairs with thymine through hydrogen bonding, forming a base pair that helps to maintain the structure and integrity of the DNA molecule. Adenine’s involvement in DNA replication is crucial for the accurate duplication of genetic information.
Anchor Text: “The Role of Adenine in DNA Replication“
Frequently Asked Questions
1. What is adenine?
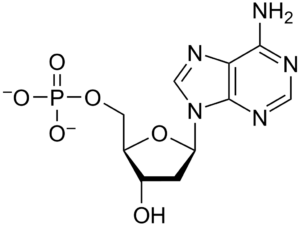
Adenine is one of the five main nucleobases found in the nucleic acids DNA and RNA. It is a purine derivative, with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate (ATP) and the cofactors nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD).
2. Is adenine a purine?
Yes, adenine is a purine. Purines are one of two types of bases found in DNA and RNA. The other type is pyrimidine.
3. How is adenine made?
Adenine is synthesized through a biochemical pathway known as the purine synthesis pathway. This involves a series of enzymatic reactions that transform simple molecules into the complex structure of adenine.
4. Is adenine and adenosine the same?
No, adenine and adenosine are not the same. Adenine is a nucleobase, while adenosine is a nucleoside consisting of adenine attached to a ribose sugar molecule.
5. Why is adenine called a nitrogenous base?
Adenine is called a nitrogenous base because it is a molecule that contains nitrogen and has the chemical properties of a base. It is one of the building blocks of DNA and RNA.
6. Why would an adenine not bond with itself?
Adenine would not bond with itself due to the specific pairing rules of DNA and RNA. In DNA, adenine always pairs with thymine, while in RNA, adenine pairs with uracil.
7. Why is adenosine used in ATP?
Adenosine is used in ATP because it can easily bind with three phosphate groups to form ATP, a molecule that cells use to store and transfer energy.
8. Why is adenine important?
Adenine is important because it is a fundamental component of nucleic acids, which are necessary for life. It plays a crucial role in the formation of DNA and RNA and is involved in protein synthesis.
9. Is adenine a nucleotide?
On its own, adenine is not a nucleotide but a nucleobase. When adenine is attached to a sugar and one or more phosphate groups, it forms a nucleotide.
10. Where is adenine found?
Adenine is found in all living cells as it is a key component of DNA and RNA. It is also present in ATP, a molecule crucial for energy transfer within cells.
Also Read: