Enzymes are “gnomes” of every cellular process. Enzymes are functional protein molecules. These biomolecules assist various metabolic reactions and participate in both anabolism and catabolism processes. They enhance the reaction rates and result in physiologically significant products.
- Dehydrogenase
- Oxidases
- Reductase
- Peroxidase
- Oxygenase
- Methyltransferase
- Acyltransferase
- Glycosyltransferase
- Transaminase
- Phosphotransferase
- Sulfur-transferase
- Nuclease
- Glycosylase
- Peptidase
- Lyase
- Isomerase
- Epimerase
- Racemase
- Mutase
- Ligase
- Kinase
- Decarboxylase
Dehydrogenase
Dehydrogenase is a type of oxidoreductase enzyme. It catalyzes the oxidation of the substrate by reducing an electron acceptor such as NAD+ or NADP++ or FAD or any flavin coenzyme. This enzyme either facilitates the removal of hydrogen to an electron acceptor along with the release of a proton or the transfer of two hydrogen atoms.
Examples- Aldehyde dehydrogenases, pyruvate dehydrogenase, succinate dehydrogenase
Oxidase
This enzyme prompts oxidation by transferring hydrogen from the substrate to the acceptor i.e., oxygen. It facilitates the oxidation of C-N and C-O bonds which are reduced to hydrogen peroxide. Examples – Xanthine oxidase, Cytochrome P450 oxidase, polyphenol oxidase.
Reductase
This enzyme catalyzes the non-reversible reduction reaction. It also acts like a dehydrogenase enzyme. Example – Nitrate reductase. It is an important enzyme for nitrogen assimilation.
Peroxidase
This enzyme also belongs to the group of oxidoreductases. It facilitates oxidation of the substrate by the involvement of hydrogen peroxide and liberates water and oxygen molecules. Most of them contain a ferric heme protein at the catalytic site. These enzymes mostly act as antioxidants. Example – Manganese peroxidase, glutathione peroxidase.
Oxygenase
This enzyme catalyzes the oxidation reaction. In this oxidation reaction, the substrate is oxidized by an oxygen atom which is obtained from molecular oxygen. Example – Tryptophan pyrrolase, tyrosinase.
Methyltransferase
This group involves enzymes that transfer methyl groups from S-adenosylmethionine (SAM) to the substrates. For example – DNA methyltransferases transfer methyl groups to cytosines.
Acyltransferase
This enzyme catalyzes the transfer of the acyl group. Example – Carnitine acyltransferases.
Glycosyltransferase
This enzyme facilitates the transfer of saccharide moieties to protein residues mostly tyrosine, serine, or threonine. These are transmembrane proteins adhered to the membranes of the Golgi apparatus.
Transaminase
This enzyme catalyzes the exchange reaction between an amino group and an alpha-keto group. It requires a coenzyme pyridoxal phosphate for its reaction.
Phosphotransferase
This enzyme is responsible for reversible phosphorylation reaction (addition of phosphate group). It transfers the phosphoryl group to hydroxyl, carboxy, and nitrogenase groups. The amino acids that are phosphorylated are serine, tyrosine and threonine.
The phosphorylase enzyme catalyzes the addition of the inorganic phosphate group to the substrate.
Sulfur transferase
These transferase enzymes are involved in the transfer of sulfur-containing groups. Example – Thiosulfate sulfurtransferase is a mitochondrial enzyme that converts cyanide to thiocyanate. The substrates of this enzyme are cyanide and thiosulfate and the products are sulfite and thiocyanate.
Nuclease
This enzyme is capable of cleaving the phosphodiester bonds present in nucleotides. It either creates a single cut or double cut. Depending upon its site of cleavage, it can be divided into endo and exonuclease. These enzymes are extensively used in biotechnology.
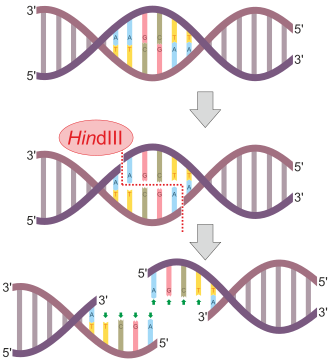
Glycosylase
This is a type of hydrolase enzyme that is involved in the hydrolysis of glycosidic bonds present between glycosyl moieties. It can be of two types depending upon the site of action i.e., O- or S- glycosides or N-glycosides.
Peptidase
Peptidase or protease undergoes proteolysis, breaking down the peptide bonds present in polypeptide chains resulting in smaller polypeptides or amino acids. It can be seven types such as serine proteases, cysteine proteases, threonine proteases, aspartic proteases, glutamic proteases, metalloproteases, and asparagine peptide lyases.
Lyase
Lyases facilitate elimination or substitution reaction along with oxidation. It cleaves C-O, C-C, C-N, C-S, and P-O bonds. Mostly these enzymes are present either as peripheral membrane proteins or as transmembrane proteins.
Isomerase
It facilitates the intramolecular structural or geometrical changes of one isomer to another isomer. The molecules are called structural isomers or stereoisomers.
Epimerase
This enzyme is a type of isomerase enzyme. It catalyzes the stereochemical inversion around an asymmetric carbon in a substrate containing more than one center of asymmetry. Such molecules are called epimers. Epimer is a pair of diastereomers.
Racemase
Contrastingly, racemases facilitate the inversion around an asymmetric carbon in a substrate with one center of asymmetry. Example – methyl malonyl-CoA epimerase.
Mutase
This is a type of isomerase enzyme that accelerates the interconversion. In this reaction, the functional groups are shifted from one position to another. Example – bisphosphoglycerate mutase, phosphoglycerate mutase.
Ligase
This enzyme helps to join molecules or large moieties by creating new bonds. The most common example is DNA ligase. It ligates various bonds such as C-O, C-S, C-N, C-C, and also nitrogen metal bonds. These remain either as peripheral or transmembrane proteins.
Kinase
This is a type of phosphotransferase enzyme where it transfers the phosphoryl group from ATP to a substrate.
Decarboxylase
This enzyme is responsible for the addition or removal of the carboxyl group. Examples are glutamate decarboxylase, histidine decarboxylase, ornithine decarboxylase, phosphoenolpyruvate carboxylase, and pyruvate decarboxylase.
What is a protein enzyme?
Metabolic pathways are solely dependent upon these protein enzymes.
Enzymes are proteinaceous molecules. These biomolecules speed up the reaction rate by minimizing the intermediate activation energy. Most enzymes are present either as peripheral membrane proteins or as transmembrane proteins. Enzymes often require a coenzyme or cofactor for their catalytic activity.
Coenzyme is a small organic molecule facilitates the transfer of the atoms such as NAD, NADPH, FAD, FMN, flavin and ATP. These enzymes can be broadly classified into six groups. (i) Oxidoreductase (ii)Transferase (iii) Hydrolases (iv) Lyases (v) Isomerases and (vi) Ligases
Protein enzyme structure
Mostly these are globular proteins.
Enzymes contain linear chains of amino acids with disulfide bonds that give rise to a three-dimensional, globular structure. The enzyme size ranges from a few amino acid residues to more than 2500 residues. However, a smaller portion of this globular structure is involved in catalytic activity.
There are binding sites that are specific to a particular substrate, cofactor, or coenzyme. The catalytic and binding sites together form the active sites of an enzyme.
Conclusion
Enzymes are globular proteins present in cells either as peripheral membrane proteins or transmembrane proteins. Along with various coenzyme and cofactors that facilitate various biochemical reactions such as oxidation-reduction reactions, elimination, substitution, and inversion reactions.
Also Read:
- Single cell plant examples
- Do fungi have enzymes
- Atp synthesis in aerobic respiration
- Genome example
- Aerobic respiration stages
- Are lysosomes organelles
- Do animal cells have flagella
- Is enzyme inhibited
- Bacteria cell wall formation
- Dna transcription diagram

I am a doctoral student of CSIR- CIMAP, Lucknow. I am devoted to the field of plant metabolomics and environmental science. I have completed my post-graduation from the University of Calcutta with expertise in Molecular Plant Biology and Nanotechnology. I am an ardent reader and incessantly developing concepts in every niche of biological sciences. I have published research articles in peer-reviewed journals of Elsevier and Springer. Apart from academic interests, I am also passionate about creative things such as photography and learning new languages.
Let’s connect over Linkedin