Phosphorane or PH5 is a very unstable chemical compound. It is a compound of pentavalent phosphorus. Let us talk about characteristics of PH5 in details below.
PH5 is a hypothetical molecule. The structure adopts trigonal bipyramidal geometry with two longer axial bonds and three comparatively short equatorial bonds. This is because of the more electron cloud pulling effect in equatorial plane the bonds become shorter.
PH5 is unstable but its derivative compound pentaphenylphosphorane is stable one.Let us discus some properties like valence electron (last orbit electron), hybridization, structure angle and other facts of PH5 lewis structure below.
How to draw PH5 lewis structure?
Very unstable PH5 lewis structure can be drawn by following the steps below where valence electrons are shown around atomic symbols of ‘P’ and ‘H’.
Step 1: counting total valence electrons
Total valence electron participated in covalent bonds of PH5lewis structure is, [5 + (1×5)] = 10. In PH5, phosphorus (P) is from group 15 and hydrogen is from group 1 in the modern Periodic table. So valence electrons can be calculated by group number of the main group element.
Step 2: choosing central atom
Phosphorus becomes the central atom in the PH5 lewis structure. Electro negativity of phosphorus (2.19) and hydrogen (2.2) are quite same. But to be a central atom one atom must contain minimum two electrons which is not possible for hydrogen atom.
Step 3: octet rule and formal charge checking
In PH5 lewis structure there is a violation of octet rule as a hypervalent molecule. That is because in the molecular orbital there are two more electrons after filling the octet configuration. But the calculated formal charge is zero for the configuration of PH5.
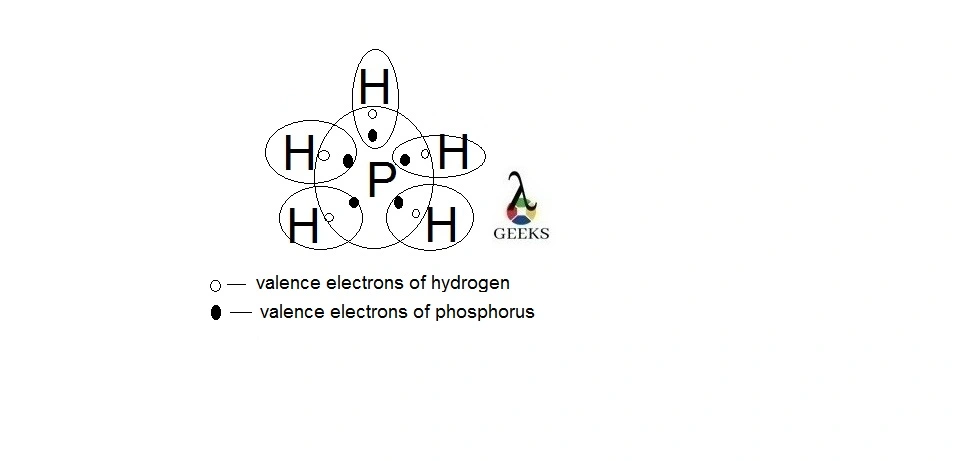
PH5 lewis structure shape
Shape of covalent molecule is known from its geometry and position of nonbonding electrons. Let us figure out PH5 lewis structure shape from hybridization.
PH5 lewis structure shape is trigonal bipyramidal similar to its geometry. This is because the central atom phosphorus has no lone pair over it. The shape of molecule is known from the sp3d hybridization. Here three hydrogen atoms connected in equatorial plane and other two in axial position.

PH5 lewis structure formal charge
Formal charge is calculated for individual atom to find energetically stable structure. Let us see PH5 lewis structure formal charge.
PH5 lewis structure’s formal charge is zero. Formal charge is measured by bonding and non bonding electron with the formula, F= (v- l- b/2) in covalent molecule. So formal charge over ‘P’ is (5- 0- 10/2) = 0 and ‘H’ is (1- 0- 2/2) = 0 in the molecule. As a whole there is no formal charge over PH5.
PH5 lewis structure angle
Structure angle of a covalent molecule can be known from the hybridization of the molecule. Let us explain PH5 lewis structure bond angle.
PH5 has two types of bond angle, one is 90 degree and another is 120 degree. Geometry of PH5 is trigonal bipyramidal so it has both axial and equatorial bonds with hydrogen. Both axial bonds make 90 degree angle with equatorial plane and angle between equatorial H-P-H bonds is 120 degree.
PH5 lewis structure octet rule
Rule of thumb Octet rule explains an atom of stabilized molecule has eight electrons in its last orbit. Let us explore if atoms of PH5 lewis structure obeys the rule.
In PH5 lewis structure extension of octet rule can be seen as it is hypervalent molecule. Phosphorus is a group 15 element which contains five electrons in last shell and needs three more electrons for octet fulfill. The molecule has excess valence electrons as “P” combines with 5 hydrogen atoms.
PH5 lewis structure lone pairs
In covalent molecule, lone pairs are the electrons of central atom which don’t participate in bond formation. Let us figure out if PH5 contains such type of electrons.
PH5 lewis structure has no lone pair. This can be understood from the hybrid molecular orbit formation. All the five electrons of sp3d orbital are paired up with 1s electron of hydrogen atoms. So there are no unshared electrons which only concentrate over parent atom.
PH5 valence electrons
Last orbit electrons are called valence electrons in an atom which can be sent to higher excited state as loosely bounded. Let us talk about valence electrons of PH5.
PH5 molecule is formed by 10 valence electrons. Phosphorus atom has five electrons in 3rd orbit and hydrogen contains one electron in its valence orbital 1s. PH5 molecule has five hydrogen atoms, so total number of outer shell valence electrons are {5+ (1×5)} = 10.
PH5 hybridization
Hybridization is intermixing of an atom’s last shell orbitals to produce energetically different shape orbital. Let us explore PH5 lewis structure hybridization.
Hybridization in PH5 lewis structure is sp3d. One 3s orbital electron of phosphorus transfers to its 3d orbital during hybrid orbital formation of central atom. Then 3s, 3p and 3d orbitals generate the new shape sp3d hybrid orbital for better overlapping with 1s hydrogen orbital.
Is PH5 ionic or covalent?
Molecular bonding is dependent on electronic configuration of atoms and force if that is electrostatic or dipole dipole. Let us explain PH5 bonds are ionic or covalent.
PH5 is a covalent compound because sp3d hybrid orbit electron cloud of phosphorus is shared with hydrogen atoms. But in case of ionic bonding, fully transferring of electron cloud between metal and non metal can be seen.
Covalent bonding of PH5 is not strong as there is energetic mismatch between sp3d hybrid orbital and 1s orbital. For the high magnitude difference the molecule become unstable one.
Conclusion:
PH5 is a covalent molecule as the bonding is formed by electron sharing between the constituent atoms. But the sharing of electron cloud between 3d and 1s orbital is not energetically supported. So the molecule does not exist in normal condition.
Also Read:
- Naoh lewis structure
- Xeo3 lewis structure
- Br2 lewis structure
- Clf2 lewis structure
- Cobr2 lewis structure
- N2o3 lewis structure
- Cai2 lewis structure
- Ibr2 lewis structure
- Sf2 lewis structure
- Of2 lewis structure

Hi…I am Triyasha Mondal, pursuing M.Sc in Chemistry. I am an enthusiastic learner. My specialization is in physical chemistry.
Let’s connect through LinkedIn: