In this article, “is MgO ionic or covalent”, covalent or ionic properties of magnesium oxide with detailed explanations are discussed briefly.
Magnesium oxide (MgO) is a solid white odorless ionic substance formed by the reaction between magnesium and oxygen with a molar mass 40.344 gm/mol and density 3.6g/cm3 by completely transferring of two electrons. It forms Magnesium hydroxide in presence of water. MgO has high melting and boiling point, 28520C and 36000C respectively.
Some evidences to prove that MgO is ionic are shown below in this article.
What is covalent and ionic compound?
Covalency in any compound arises due to sharing of electron cloud between two consisting atoms and ionic behaviour arises due to complete transferring of electrons.
In a covalent compound electron cloud is shifted to comparatively more electronegative atom but not completely transferred from one atom to another one. But shifting of electron cloud depends upon the corresponding electronegativity of the participating atoms. Bonding electrons are shifted more towards the more electronegative atom.
In ionic species, electrons are completely donated by the more electropositive atom to the more electronegative atom due to difference of electronegativity.
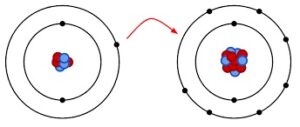
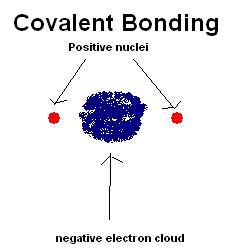
Image Credit: Wikipedia
To know more please check: 10 Ionic Bond Examples: Explanation And Detailed Facts
How is MgO ionic?
MgO is definitely an ionic compound. The difference in electronegativity between magnesium and oxygen is relatively high than any two atoms in covalent compound. Mg is basically an electropositive alkaline earth metal and oxygen is one of the most electronegative (next to fluorine) molecular species.
Mg has atomic number 12 (1s2 2s22p63s2) and has two electrons in its outer most electron shell (valance shell). It donates its two valance electrons to oxygen and achieves the nearest noble gas electronic configuration that is Neon (1s2 2s22p6). After donating two electrons Mg becomes dipositive Mg2+, a stable closed shell configuration.
Oxygen has total eight electrons (1s2 2s2 2p4) and has four electrons in its valence shell. After accepting four electrons from Mg, oxygen becomes O2- and gains the nearest noble gas electron configuration (1s2 2s2 2p6) and stability is increased.
% of Ionic character (I.C) can be calculated from the following formula-
% I.C =16 ( χA – χB) + 3.5 ( χA – χB)2 .In this formula χA and χB are the electronegativity of species A and B respectively.
- % I.C = 16 (3.44-1.31) + 3.5 (3.44-1.31)2
- % I.C = 50
The electronegativity of Mg and oxygen is 1.31 and 3.44 in pauling scale respectively. This high value of percentage of ionic character indicates that the magnesium oxide cannot be a covalent compound.
To know more please follow: 15 Coordinate Covalent Bond Examples: Detailed Insight And Facts
Why MgO is not a covalent compound?
In magnesium oxide two electrons from magnesium is completely transferred to the valence shell of oxygen and make the octet filled up. Mg becomes Mg2+ after donating two electrons to oxygen. But in covalent compound, electron cloud is shifted from less electronegative atom to more electronegative atom but not completely transferred.
Difference of electronegativity between magnesium and oxygen is (3.44-1.31) = 2.13 in Pauling scale. This difference in electronegativity indicates that MgO cannot be a covalent compound. Besides that, due to +2 and -2 charge on Mg and O, MgO has greater amount of lattice energy.
To know more please check: 4 Single Covalent Bond Examples : Detailed Insights And Facts
MgO Lewis Structure
Lewis structure is invented by Gilbert N. Lewis in 1916 in his article named as “The atom and the molecule”. Lewis structure is basically the simplified representation of valance shell electrons around the respective atoms. The number of dots around any atom indicates the number of valance electrons.
Lewis dot structure is applied to illustrate the formation of cation or anion by donating or accepting the electrons. But it should be kept in mind that lewis dot structure of any molecule cannot detect the geometry or shape of that molecule. Though this representation is used to make sure that the respective atoms in that molecule satisfy the octet rule or not and formal charge of each of the atoms can also be determined from lewis structure.
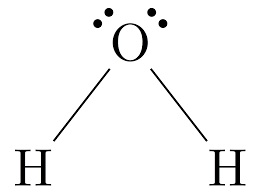
In the above image, lewis dot structure of water is shown. Outer most shell electrons of oxygen are shown around oxygen atoms.
In the lewis structure of MgO, Mg has two valance shell electrons and oxygen has six valance shell electrons. Mg donates its two valance electrons to make the electron configuration 2,8 and oxygen accepts those two electrons and gains the configuration 2,8,8. The valance electrons and the transferred electrons are shown in the following lewis dot structure image of MgO.

To know more please go through: 5 Polar Covalent Bond Examples: Detailed Insights And Facts
Frequently Asked Questions (FAQ)
How MgO is formed?
Answer: Magnesium reacts with oxygen and form the whitish powdered compound, MgO through an exothermic reaction and produce a large amount of energy in form of light.
What happens when MgO reacts with water?
Answer: When MgO comes in contact with water, magnesium hydroxide [Mg(OH)2] is formed through a reversible reaction. MgO + H2O → Mg(OH)2
Why MgO is not soluble in water?
Answer: MgO has a huge amount of lattice energy due to strong ionic interaction between Mg2+ and O2-. Hydration energy or solvation energy cannot exceed the strong lattice energy. Thus, MgO is not soluble in water.

Hello,
I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202