First order reaction is linearly dependent on the reactant concentration of only one reactant. In this article, “first order reaction example”, different types of examples with detailed explanations are discussed briefly.
The examples are-
- Hydrolysis of aspirin.
- Reaction of t-butyl bromide with water
- Hydrolysis of anti cancer drug Cis-platin
- Decomposition of hydrogen peroxide
- Hydrolysis of methyl acetate
- Hydrogenation of ethene
- Hydrolysis of sucrose.
- Decomposition of ammonium nitrate.
- Decomposition of dinitrogen pentoxide.
- Decomposition of sulfuryl chloride
- Decomposition of azomethane
What is First Order Reaction?
First order reaction kinetics is defined as one type of chemical reaction in which the rate of the reaction is dependent only first power of a single reactant.
Rate law of a first order reaction is written as -d[A]/dt. [A] is the concentration of reactant A, t is time and -d[A]/dt is the rate of concentration with respect to tome.
Rate equation of first order kinetics is-
A= A0 exp(-kt)
ln A=ln A0 -kt.
[A= concentration at time t; A0= concentration of reactant at time, t=0; k= first order rate constant and t= time].The unit of the first order rate constant is time-1.
The rate of the reaction only depends on the reactant concentration proportionally. The plot ln A with time is drawn below. It obeys the equation of a straight line (y= mx +c)


Hydrolysis of Aspirin
Hydrolysis of aspirin is a special type of first order reaction that is defined as pseudo first order reaction. Aspirin undergoes hydrolysis (reaction with water) breaks down into two components, acetic acid (CH3COOH)and salicylic acid. reaction is written as-
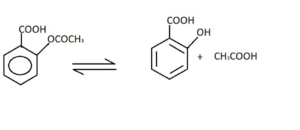

Reaction of t-butyl bromide with water
This reaction is nucleophilic substitution unimolecular or SN1 type reaction. Bromide is eliminated as a leaving group forming a stable 30 carbocation. Then water molecule attacks the carbocation and form t-butyl alcohol as the substitution product.
Hydrolysis of anti-cancer drug, Cis-platin
Cis-platin, cis-[Pt(NH3)2Cl2] is a very well known anti-cancer drug. Cis-platin undergoes hydrolysis reaction mainly in Pt-Cl bonds and form Pt-aqua complex. Activation energy required for this hydrolysis is approximately 25-27 kcal/mol and free energy change is 0-2 kcal/mol. The rate law of this reaction can be explained through first order reaction kinetics.
To know more please go through: N2 polar or nonpolar: Why, How, Characteristics, And Detailed Facts
Decomposition of hydrogen peroxide
Hydrogen peroxide undergoes decomposition reaction but this process is very much slow under moderate temperature. The rate of the reaction can be accelerated in high temperature or in presence of catalyst (manganese oxide or lead oxide). This hydrolysis products are water and oxygen.
2H2O2 → 2H2O + O2
Hydrolysis of methyl acetate
Methyl acetate takes part in hydrolysis in presence of large concentration of acid (HCl) in the range of 90-1100C. Large concentration of reactant indicates the reaction to proceed through pseudo first order reaction. It produces acetic acid and methyl alcohol as the product. The first order rate constant is 0.17×10-8 s-1 at 250C in absence of any catalyst and this rate constant value will be 1.41×10-4 (mol/L) s-1 in presence of catalyst.
CH3COOCH3 +H2O → CH3OH + CH3COOH
To know more please check: Is ch2cl2 Polar: Why, How, When and Detailed Facts
Hydrogenation of ethene
The reaction of ethene with hydrogen in presence of finely divided nickel catalyst is mainly a first order reaction. Activation energy is required for the reaction is 15.8 KJ/mol and temperature 1500C. This hydrogenation produces ethane. C=C double bond is reduced to form C-C single bond.
CH2=CH2 + H2 → CH3CH3
Hydrolysis of sucrose
Sucrose undergoes hydrolysis reaction in which the glycosidic linkage is broken and form glucose and fructose. This is also an example of pseudo first order reaction because the change of concentration of water is very much negligible before and after completion of the reaction. In absence of enzyme, this reaction proceeds in very slow motion, but in presence of catalyst the rate will be faster.
C12H22O11 +H2O → C6H12O6+ C6H12O6
Decomposition of ammonium nitrate
Heat can cause the decomposition in solid ammonium nitrate below 3000C. This decomposition produces nitrous oxide (N2O) and water.
NH4NO3 → N2O + 2H2O.
This decomposition reaction follows the first order kinetics.
At more than 3000C the decomposition reaction is totally different. The reaction is-
2NH4NO3 → 2N2+ O2 + 4H2O
It also undergoes in first order kinetics.
Decomposition of dinitrogen pentoxide
Dinitrogen pentoxide (N2O5) undergoes decomposition reaction and follows first order kinetics. The first order rate constant is 5×10-4 s-1 at 450C.
2N2O5(g) → 4NO2(g) + O2(g)
To know more please follow: 15 Coordinate Covalent Bond Examples: Detailed Insight And Facts
Decomposition of sulfuryl chloride
Sulfuryl chloride (SO2Cl2) undergoes decomposition reaction in presence of heat above 373K (bp- 303K). This decomposition is an example of first order homogenous gas phase reaction and produces sulfur dioxide (SO2) and chlorin (Cl2).
For this decomposition reaction older sample of sulfuryl chloride becomes yellowish colour.
SO2Cl2 → SO2+ Cl2
Decomposition of azomethane
The decomposition reaction is carried out in basically higher temperature (290-3400C). Azomethane undergoes decomposition and follows first order rate law. Nitrogen and ethane are obtained as the decomposed product.
(CH3)2N2 → N2 + C2H6
The total pressure of azomethane at the starting of the reaction is 36.2 mm of Hg and after 15 min it becomes 42.4 mm of Hg.
To know more please follow: SN2 Examples: Detailed Insights And Facts
Frequently Asked Questions (FAQ)
What is the half time of a first order reaction?
Answer: Half life of any reaction is the time in which the reaction is 50% completed. The formula of half life of a first order reaction is t1/2 = 0.693/k [k = first order rate constant]. Reactant concentration have no effect on the half lifr of a first order reaction rate.
How much time will be required for the completion of a first order reaction?
Answer: It takes infinite time to complete any first order reaction. Basically, zero order reaction is completed within finite time. Reaction having order more than zero takes infinite time to be completed.
What is the nature of the graph plotting reactant concentration vs time?
Answer: The nature of the graph plotting reactant concentration vs time is hyperbolic in nature.
In which factor any first order equation depends?
Answer: Reactant concentration is one and only determining factor of a first order reaction. If the concentration of reactant becomes double then the first order reaction rate will be doubled.
Also Read:
- Redox reactions
- How to balance redox reaction
- Neutralization reaction
- Addition reaction example
- Light independent reaction example
- Exchange reaction example
- Exothermic reaction 3
- Metathesis reaction
- Knoevenagel reaction
- Light dependent reaction

Hello,
I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202