In this article “decomposition reaction example” different types of examples with detailed explanations are discussed below.
The name of the examples is written below-
- Decomposition of Hydrogen Peroxide
- Decomposition of Carbonic Acid
- Electrolysis of Water
- Decomposition of Ferrous Sulphate Crystals
- Decomposition of Potassium Chlorate
- Decomposition of Ferric Hydroxide
- Decomposition of Oxalic Acid
- Decomposition of Limestone
- Decomposition of Silver Bromide
- Decomposition of Lead Nitrate.
- Decomposition of Starch
- Decomposition of Ozone
Decomposition Reaction
A decomposition reaction is defined as one type of chemical reaction when one component breaks down into its constituent components. Decomposition reaction is exactly opposite of the chemical synthesis reaction.
This can be classified into two types.
- Physical Decomposition
- Chemical Decomposition
Physical decomposition is generally reversible (some irreversible physical decomposition is also there) and only physical properties (phase, melting or boiling temperature) are changed during this type of decomposition. For example- boiling of water, melting of an ice cube etc.
But most of the chemical decomposition is irreversible and new products, having totally different characteristics from reactant, are formed after the decomposition.
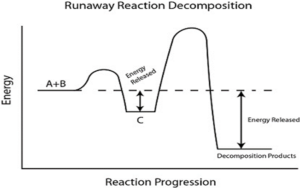
Image Credit: Wikimedia Commons
Types of Decomposition Reaction
Three types of decomposition reaction is known in Chemistry. They are-
- Thermal decomposition
- Electrolytic decomposition
- Photochemical decomposition
To know more please follow: SN2 Examples: Detailed Insights And Facts
Decomposition of Hydrogen Peroxide
The chemical reactivity of hydrogen peroxide is very much high. Thus it is easily decomposed in presence of light. To avoid the decomposition, it is kept in a dark bottle.
Water and oxygen are obtained as the decomposition product of hydrogen peroxide.
The balanced equation is-
2H2O2→ 2H2O + O2
Decomposition of Carbonic Acid
Carbonic acid is a weak acid and an ingredient of soft drinks in which carbon dioxide is dissolved in the solution at high pressure.
In chemical decomposition of carbonic acid carbon dioxide and water molecule is formed as the product.
H2CO3→ H2O + CO2
To know more please go through : SN1 mechanism: Detailed Insights And Facts
Electrolysis of Water
When electric current passes through water then it breaks down into its two constituent atoms, hydrogen and oxygen gas are evolved.
The balanced equation is-
2H2O → 2H2 + O2
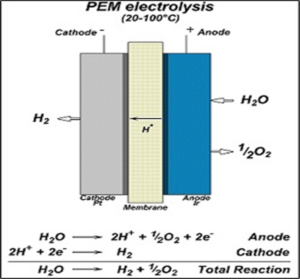
Image Credit: Wikimedia Commons
Decomposition of Ferrous Sulphate Crystals
Ferrous sulphate crystals contain water molecule. On heating, the water molecule is removed from the crystal and anhydrous ferrous sulphate is formed. If the temperature of reaction medium is high then anhydrous ferrous sulphate undergoes further decomposition and forms ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3). As a result, a smell of burning sulphur comes out from the reaction medium.
The balanced equation is-
FeSO4. 7H2O → FeSO4 + 7H2O
2FeSO4→ Fe2O3 (s) + SO2(g) + SO3(g)
Decomposition of Potassium Chlorate
Thermal decomposition of potassium chlorate undergoes in irreversible pathway. This reaction can be accelerated using catalyst (manganese dioxide) and forms potassium chloride and oxygen gas.
The balanced equation of the decomposition is-
2KClO3(s) → 2KCl (s) + 3O2 (g)
To know more please follow : Peptide Bond vs Disulfide Bond: Comparative Analysis and Facts
Decomposition of Ferric hydroxide
Ferric hydroxide undergoes chemical decomposition in presence of heat energy and form ferric oxide and water as product. It is also an irreversible reaction.
The balanced equation is written below-
2Fe (OH)3→ Fe2O3 + 3H2O
Decomposition of Oxalic Acid
The thermal decomposition reaction is carried out in presence of concentrated sulphuric acid and forms carbon monoxide (CO), carbon dioxide (CO2) and water molecule (H2O). The amount of activation energy is required is almost 18.6 kcal/mol
The balanced equation is-
(COOH)2 →CO + CO2 +H2O
Decomposition of Limestone
Calcium oxide (CaO) is obtained from the thermal decomposition reaction of limestone, containing calcium carbonate (CaCO3). This decomposition is carried out at high temperature and low temperature (below the melting point).
The balanced equation of the above decomposition is-
CaCO3(s) → CaO(s)+ CO2(g)
Decomposition of Silver Bromide
Silver bromide (AgBr) undergoes decomposition reaction in presence of light (photochemical decomposition). When AgBr is exposed in sunlight the decomposition reaction has started and silver is accumulated as metal silver and bromine gas is liberated.
The balanced equation of this photochemical decomposition is-
2AgBr → 2Ag(s) +Br2(g)
To know more please check: 5+ Metallic Bond Examples: Explanation and detailed Facts
Decomposition of Lead Nitrate
Lead nitrate undergoes decomposition reaction in presence of heat. Lead oxide (PbO), nitrogen dioxide (NO2) and oxygen (O2) are obtained as the decomposed product of lead nitrate.
The balanced equation of the decomposition is-
2PbNO3→ 2PbO + 4NO2 + O2
Decomposition of Starch
Decomposition of starch is one of the most important chemical reactions in living organism. Starch is a complex form of sugar molecule. So, on decomposition of starch sugar molecules, in most of the cases maltose and smaller amount of glucose is produced.
Decomposition of Ozone
Decomposition of ozone undergoes in presence of ultra violet ray (λ=2537 A0) in atmosphere. The partial pressure of ozone is one of the most important determining factors in this photochemical decomposition. It is an example of chain reaction.
The balanced equation is-
O3 → O2+ O
O2+O → O3
O+O3→ O2+O2
Frequently Asked Questions (FAQ)
Is decomposition reaction exothermic or endothermic?
Answer: Decomposition reaction is mainly breaking down of a bigger molecule into two or more smaller molecules. This requires a large amount of energy. So, in most of the cases decomposition is endothermic in nature, but some exothermic decomposition reaction is also there.
What is another name of decomposition reaction?
Answer: Decomposition reaction is also called as chemical analysis or chemical breakdown.
What is the significance of decomposition reaction?
Answer: One of the applications of decomposition reaction is extraction metals from their respective ores. For example, metal zinc is collected from its ore calamine through decomposition reaction.
Also Read:
- Exergonic reaction
- Addition reaction example
- Exothermic reaction 3
- Oxidation reaction
- Mitsunobu reaction
- Kolbe reaction
- Endergonic reaction example
- Nuclear fission reaction
- How to balance redox reaction
- Exchange reaction example

Hello,
I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202