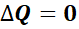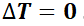When it comes to electric motors, the choice between outrunner and inrunner configurations is a crucial decision for electronics students. These two motor types have distinct advantages and disadvantages, and understanding their technical specifications is essential for designing and implementing efficient electrical systems. This comprehensive guide will delve into the intricate details of outrunner and inrunner motors, providing electronics students with a thorough understanding of their performance characteristics, theoretical principles, and practical applications.
Inrunner Motors: High-Speed Efficiency
Inrunner motors, also known as “in-hub” or “spindle” motors, are characterized by their rotor positioned inside the stator. The stator consists of multiple permanent magnets, while the rotor contains copper windings. This unique configuration offers several advantages:
- High RPM Capability: Inrunner motors are capable of reaching exceptionally high rotational speeds, making them ideal for applications that require rapid movements, such as drones and high-speed RC vehicles.
- Efficient Heat Dissipation: The close proximity of the windings to the air-cooled exterior of the motor allows for efficient heat dissipation, improving overall efficiency and performance.
- Compact Design: Inrunner motors have a more compact and lightweight design compared to their outrunner counterparts, making them suitable for applications where space and weight are critical factors.
- High-Speed Performance: The high-speed capabilities of inrunner motors make them well-suited for tasks that demand rapid acceleration and precise control, such as in robotics and industrial automation.
- Lower Torque Output: While inrunner motors excel in high-speed applications, they generally have a lower torque output compared to outrunner motors.
Outrunner Motors: High-Torque Powerhouses
Outrunner motors, also known as “external rotor” or “hub” motors, have the rotor positioned outside the stator. The stator consists of copper windings, while the rotor contains permanent magnets. This configuration offers several distinct advantages:
- High Torque Output: Outrunner motors are designed to provide exceptional low-RPM torque, making them ideal for applications that require high-torque, low-speed performance, such as electric bicycles and electric scooters.
- Larger Diameter: Outrunner motors typically have a larger diameter compared to inrunner motors of similar power output, allowing for a more efficient conversion of electrical energy into mechanical energy.
- Lower RPM Capability: While outrunner motors excel in low-speed, high-torque applications, they generally have a lower top speed compared to inrunner motors.
- Less Efficient Heat Dissipation: Due to their larger size and the increased distance between the windings and the air-cooled exterior, outrunner motors can be less efficient in dissipating heat, which can impact their overall performance at higher RPMs.
- Bulkier Design: Outrunner motors have a larger and heavier design compared to inrunner motors, which can be a consideration in applications where size and weight are critical factors.
Technical Specifications Comparison
To further understand the differences between outrunner and inrunner motors, let’s delve into their technical specifications:
| Specification | Inrunner Motors | Outrunner Motors |
|---|---|---|
| Size and Weight | Compact and lightweight | Larger diameter and heavier |
| Speed and Torque | Higher RPM capability, lower torque output | Lower RPM capability, higher torque output |
| Efficiency | Generally more efficient due to compact design and efficient heat dissipation | Can be less efficient, especially at higher RPMs, due to larger size and less efficient heat dissipation |
| Applications | Ideal for high-speed applications (e.g., drones, RC cars) | Suitable for high-torque, low-speed applications (e.g., electric bicycles, electric scooters) |
Theoretical Principles and Electronics Formulas
The fundamental principle governing the operation of both inrunner and outrunner motors is the principle of electromagnetic induction. This principle states that a voltage is induced in a conductor moving relative to a magnetic field, which is the foundation for the operation of all electric motors.
The formula for calculating the power (P) of an electric motor is:
P = τ × ω
Where:
– P = Power (Watts)
– τ = Torque (Nm)
– ω = Angular velocity (rad/s)
This formula demonstrates the relationship between power, torque, and angular velocity, which is crucial for understanding the performance characteristics of both inrunner and outrunner motors.
Practical Examples and Numerical Problems
Let’s explore some practical examples and numerical problems to further illustrate the differences between inrunner and outrunner motors.
Example 1: Calculating the power of an inrunner motor
Given:
– Torque (τ) = 0.1 Nm
– Angular velocity (ω) = 500 rad/s
P = τ × ω
P = 0.1 Nm × 500 rad/s
P = 50 Watts
Example 2: Calculating the torque of an outrunner motor
Given:
– Power (P) = 100 Watts
– Angular velocity (ω) = 200 rad/s
P = τ × ω
100 Watts = τ × 200 rad/s
τ = 0.5 Nm
These examples demonstrate how the power formula can be used to determine the power and torque characteristics of inrunner and outrunner motors, respectively.
Figures, Data Points, and Measurements
To provide a visual representation of the differences between outrunner and inrunner motors, consider the following figures, data points, and measurements:
- Size Comparison:
-
A 500W outrunner motor may have a diameter of 60mm, while a 500W inrunner motor may have a diameter of 40mm.
-
Weight Comparison:
-
A 500W outrunner motor may weigh 1kg, while a 500W inrunner motor may weigh 0.5kg.
-
Speed and Torque Curves:
- Inrunner motors typically have a higher RPM capability and lower torque output, while outrunner motors provide better low-RPM torque and lower top speeds.
These data points and measurements can help electronics students visualize the physical and performance differences between outrunner and inrunner motors, aiding in their understanding of the technical specifications and practical applications of these motor configurations.
Conclusion
In the world of electric motors, the choice between outrunner and inrunner configurations is a crucial decision for electronics students. Inrunner motors excel in high-speed applications, offering efficient heat dissipation and compact design, while outrunner motors are better suited for high-torque, low-speed tasks, with their larger diameter and higher low-RPM torque output.
By understanding the technical specifications, theoretical principles, and practical examples of these two motor types, electronics students can make informed decisions when designing and implementing efficient electrical systems. This comprehensive guide has provided a detailed exploration of the key differences between outrunner and inrunner motors, equipping you with the knowledge and tools necessary to navigate the world of electric motor technology.
References
- In-runner VS Out-runner .. inherent Advantages/Disadvantages – Endless Sphere Forum
- Inrunner vs. Outrunner – RCPowers Forum Discussion
- Inrunner vs. outrunner – RC Groups
- Inrunner Vs Outrunner – Reacher Technology Co.,Ltd
- Outrunner vs inrunner motors – Reddit