Endonucleases are a class of enzymes that play a crucial role in DNA and RNA metabolism. These enzymes are responsible for cleaving the phosphodiester bonds within the nucleic acid chains, resulting in the fragmentation of the DNA or RNA molecule. Endonucleases are found in various organisms, including bacteria, archaea, and eukaryotes, and they are involved in important biological processes such as DNA repair, replication, recombination, and gene expression. They are highly specific in their recognition and cleavage of nucleic acids, and their activity can be influenced by factors such as sequence specificity, cofactors, and the presence of other proteins. Understanding the mechanisms and functions of endonucleases is essential for unraveling the complexities of genetic processes and developing applications in biotechnology and medicine.
Key Takeaways
- Endonuclease enzymes are enzymes that cleave DNA or RNA at specific internal sites.
- They play a crucial role in DNA repair, replication, and recombination.
- Examples of endonuclease enzymes include restriction enzymes, CRISPR-associated endonucleases, and DNA repair enzymes.
- Endonuclease enzymes are widely used in molecular biology research and biotechnology applications, such as gene editing and DNA sequencing.
Restriction Endonuclease Enzyme Example
T7 endonuclease (P00641)
T7 endonuclease is an example of a restriction endonuclease enzyme. It is derived from the bacteriophage T7, a virus that infects bacteria. The T7 endonuclease enzyme is responsible for cleaving DNA at specific recognition sites. It recognizes and cuts DNA sequences that are palindromic, meaning they read the same on both strands when oriented in the 5′ to 3′ direction.
The T7 endonuclease enzyme is widely used in molecular biology research, particularly in DNA sequencing and genetic engineering. It is commonly employed in techniques such as DNA footprinting, where it is used to identify protein binding sites on DNA. The T7 endonuclease enzyme’s ability to cleave DNA at specific sites makes it a valuable tool in various applications.
T4 endonuclease II (P07059)
T4 endonuclease II is another example of a restriction endonuclease enzyme. It is derived from the bacteriophage T4, which infects Escherichia coli bacteria. Similar to the T7 endonuclease, T4 endonuclease II recognizes and cleaves DNA at specific recognition sites. It also recognizes palindromic DNA sequences and cuts the DNA strands at these sites.
T4 endonuclease II has been extensively studied and is known for its role in DNA repair processes. It is involved in the repair of DNA damage caused by ultraviolet (UV) radiation. The enzyme recognizes and cleaves damaged DNA, allowing for the removal and replacement of the damaged DNA segment. This repair mechanism helps maintain the integrity of the genetic material and ensures proper cellular functioning.
Bal 31 endonuclease
Bal 31 endonuclease is a type of exonuclease enzyme that acts on both DNA and RNA molecules. Unlike the previous examples, Bal 31 endonuclease does not cleave DNA at specific recognition sites. Instead, it degrades DNA or RNA molecules starting from the ends. This enzyme is commonly used in molecular biology research to generate DNA fragments of specific lengths.
Bal 31 endonuclease is particularly useful in DNA sequencing applications, where it is used to generate DNA fragments for analysis. It can be used to create nested deletions, where sequential digestion with Bal 31 endonuclease generates a series of DNA fragments with progressively shorter lengths. These fragments can then be sequenced to determine the nucleotide sequence of the original DNA molecule.
Endonuclease I (endo I; P25736)
Endonuclease I, also known as endo I, is an example of a restriction endonuclease enzyme found in Escherichia coli bacteria. It recognizes and cleaves DNA at specific recognition sites. Endo I recognizes palindromic DNA sequences and cleaves the DNA strands within these sequences.
Endo I has been extensively studied and is known for its role in DNA repair processes. It is involved in the repair of DNA damage caused by oxidative stress. The enzyme recognizes and cleaves damaged DNA, allowing for the removal and replacement of the damaged DNA segment. This repair mechanism helps maintain the integrity of the genetic material and protects the cell from oxidative damage.
Micrococcal nuclease (P00644)
Micrococcal nuclease is an enzyme derived from the bacterium Staphylococcus aureus. It is a non-specific endonuclease that cleaves DNA and RNA at multiple sites. Unlike the previous examples, micrococcal nuclease does not recognize specific DNA sequences. Instead, it cleaves DNA and RNA molecules at random positions.
Micrococcal nuclease is commonly used in molecular biology research for various applications. It is often employed to digest chromatin, the complex of DNA and proteins that make up the chromosomes. The enzyme can selectively digest the DNA regions that are not bound to proteins, allowing for the study of protein-DNA interactions and chromatin structure.
Endonuclease II (endo VI, exo III; P09030)
Endonuclease II, also known as endo VI or exo III, is an example of a restriction endonuclease enzyme found in Escherichia coli bacteria. It recognizes and cleaves DNA at specific recognition sites. Endo II recognizes palindromic DNA sequences and cleaves the DNA strands within these sequences.
Endo II has been extensively studied and is known for its role in DNA repair processes. It is involved in the repair of DNA damage caused by various factors, including chemical agents and radiation. The enzyme recognizes and cleaves damaged DNA, allowing for the removal and replacement of the damaged DNA segment. This repair mechanism helps maintain the integrity of the genetic material and ensures proper cellular functioning.
S1 nuclease (P24021)
S1 nuclease is an enzyme derived from the fungus Aspergillus oryzae. It is a single-strand-specific endonuclease that cleaves single-stranded DNA and RNA molecules. S1 nuclease does not recognize specific DNA or RNA sequences but cleaves the phosphodiester bonds in single-stranded nucleic acids.
S1 nuclease is commonly used in molecular biology research for various applications. It is often employed to remove single-stranded DNA or RNA molecules from a mixture. The enzyme can selectively degrade the single-stranded nucleic acids, leaving behind the double-stranded DNA or RNA molecules intact.
P1-nuclease (P24289)
P1-nuclease is an enzyme derived from the bacterium Penicillium citrinum. It is a non-specific endonuclease that cleaves both single-stranded and double-stranded DNA and RNA molecules. P1-nuclease does not recognize specific DNA or RNA sequences but cleaves the phosphodiester bonds in nucleic acids.
P1-nuclease is commonly used in molecular biology research for various applications. It is often employed to digest DNA or RNA molecules, generating smaller fragments for analysis. The enzyme can be used to generate specific DNA or RNA fragments of desired lengths, allowing for the study of nucleic acid structure and function.
Mung bean nuclease I
Mung bean nuclease I is an example of an endonuclease enzyme that plays a crucial role in DNA cleavage. This enzyme, also known as J. DNase I (P00639), is derived from the mung bean plant (Vigna radiata). It is widely used in molecular biology research and various applications due to its ability to cleave DNA at specific sites.
Structure and Mechanism
Mung bean nuclease I is a single-chain protein with a molecular weight of approximately 34 kDa. It consists of 305 amino acids and forms a compact globular structure. The enzyme contains two calcium ions that are essential for its catalytic activity.
The mechanism of action of mung bean nuclease I involves the recognition and cleavage of DNA at specific sites. It binds to the DNA molecule and creates a nick, resulting in the formation of a 3′-OH and a 5′-phosphate group. This cleavage activity makes it a valuable tool in various molecular biology techniques, such as DNA sequencing and cloning.
Specificity and Cleavage Activity
Mung bean nuclease I exhibits a high degree of specificity in recognizing and cleaving DNA. It primarily targets single-stranded DNA and RNA molecules, making it useful for applications that involve the removal of unwanted nucleic acids. The enzyme shows a preference for cleaving DNA at sites containing pyrimidine-rich sequences.
The cleavage activity of mung bean nuclease I is temperature-dependent. It is most active at temperatures ranging from 30 to 37 degrees Celsius. This optimal temperature range allows for efficient DNA cleavage while maintaining the stability of the enzyme.
Applications
Mung bean nuclease I finds widespread use in molecular biology research and various applications. Some of its key applications include:
-
Removal of RNA: The enzyme is commonly used to remove RNA contaminants from DNA samples. It selectively degrades RNA molecules, leaving behind pure DNA for downstream applications.
-
DNA sequencing: Mung bean nuclease I is employed in DNA sequencing protocols to remove single-stranded DNA regions and improve sequencing accuracy.
-
Cloning: The enzyme is utilized in cloning experiments to remove unwanted DNA fragments and generate clean DNA inserts for ligation into vectors.
-
Mapping DNA-protein interactions: Mung bean nuclease I is employed to map protein-binding sites on DNA molecules. By cleaving DNA at specific protein-DNA interaction sites, researchers can identify and study these interactions.
Mung bean nuclease I is an important example of an endonuclease enzyme. Its specific cleavage activity and high degree of specificity make it a valuable tool in various molecular biology techniques. From removing RNA contaminants to mapping DNA-protein interactions, this enzyme has a wide range of applications in the field of molecular biology. Researchers continue to explore its potential and develop new uses for this versatile enzyme.
When Restriction Enzymes Were First Isolated in 1970
In the field of molecular biology, the discovery of restriction enzymes, also known as endonucleases, in 1970 was a groundbreaking moment. These enzymes revolutionized the way scientists manipulate and study DNA. Before their discovery, scientists faced numerous challenges when it came to manipulating DNA molecules. However, the isolation of restriction enzymes opened up a whole new world of possibilities.
Restriction enzymes are naturally occurring proteins that are found in bacteria and archaea. They play a vital role in these organisms’ defense mechanisms against foreign DNA, such as viral DNA. These enzymes have the remarkable ability to recognize specific DNA sequences and cut the DNA at those sites. This ability to cleave DNA at specific locations is what makes restriction enzymes so valuable in molecular biology.
The discovery of restriction enzymes was a result of the pioneering work of scientists Werner Arber, Hamilton O. Smith, and Daniel Nathans. Arber was the first to propose the existence of these enzymes, while Smith and Nathans were the ones who successfully isolated and characterized the first restriction enzyme, called HindII. This enzyme was isolated from the bacterium Haemophilus influenzae.
The isolation of HindII was a significant breakthrough because it demonstrated that restriction enzymes could be purified and used as tools for manipulating DNA. This discovery paved the way for the development of numerous techniques in molecular biology, such as DNA cloning, DNA sequencing, and genetic engineering.
The isolation of restriction enzymes opened up a whole new world of possibilities for scientists. They could now cut DNA molecules at specific sites, allowing them to study and manipulate genes with precision. This breakthrough not only revolutionized the field of molecular biology but also had a profound impact on various other scientific disciplines, including medicine, agriculture, and biotechnology.
Where Are Endonucleases Found
Endonucleases are enzymes that play a crucial role in DNA and RNA metabolism. These enzymes are found in various natural sources, including both living organisms and laboratory settings. Let’s explore some of the common sources of endonucleases:
Natural sources of endonucleases
-
Living organisms: Endonucleases are naturally present in a wide range of living organisms, including bacteria, archaea, fungi, plants, and animals. These enzymes are essential for DNA repair, replication, and recombination processes. In humans, endonucleases are involved in DNA repair mechanisms, ensuring the integrity of our genetic material.
-
Microorganisms: Many microorganisms, such as bacteria and viruses, produce endonucleases as part of their defense mechanisms. These enzymes help microorganisms fight against foreign DNA, such as viral DNA, by cleaving it into smaller fragments. This defense mechanism prevents the foreign DNA from replicating and causing harm to the microorganism.
-
Restriction enzymes: Restriction enzymes are a specific type of endonucleases that are commonly found in bacteria. These enzymes play a vital role in the bacterial immune system, protecting them from viral infections. Restriction enzymes recognize specific DNA sequences and cleave the DNA at or near these sequences. This process is known as restriction digestion and is widely used in molecular biology research.
Bacterial and viral origins of endonucleases
-
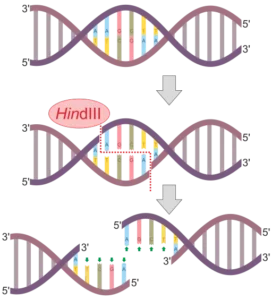
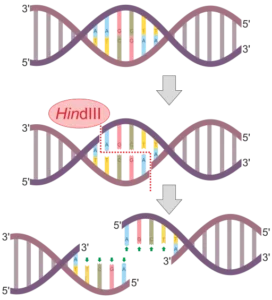
Bacterial origins: Bacteria are a rich source of endonucleases, with various types and specificities. Restriction enzymes, as mentioned earlier, are one example of endonucleases derived from bacteria. These enzymes are named after the bacterial species from which they were first isolated. For example, EcoRI is derived from Escherichia coli, and HindIII is derived from Haemophilus influenzae. Bacterial endonucleases have been extensively studied and used in molecular biology techniques, such as DNA cloning and genetic engineering.
-
Viral origins: Viruses are another source of endonucleases, which they utilize as part of their replication process. These viral endonucleases help the virus cleave host DNA, allowing the viral genetic material to integrate into the host genome. Some viral endonucleases have been studied for their potential applications in gene therapy and targeted genome editing.
How Do Endonucleases Cut DNA?
Endonucleases are enzymes that play a crucial role in DNA cleavage. They are responsible for breaking the phosphodiester bonds that hold the DNA strands together. This process is essential for various biological processes, including DNA repair, replication, and recombination. Let’s explore how endonucleases accomplish this task and the specificity they exhibit in DNA cleavage.
Cleavage of Phosphodiester Bond
The cleavage of the phosphodiester bond is a fundamental step in DNA cleavage by endonucleases. The phosphodiester bond is the chemical bond that connects the nucleotides in a DNA strand. Endonucleases have the ability to break this bond, resulting in the separation of the DNA strands.
When an endonuclease encounters a specific DNA sequence, it binds to that sequence and forms a complex with the DNA. Within this complex, the endonuclease positions itself at a specific site along the DNA strand, known as the cleavage site. At this site, the endonuclease catalyzes the hydrolysis of the phosphodiester bond, leading to the cleavage of the DNA strand.
Specificity of Endonucleases in DNA Cleavage
One remarkable aspect of endonucleases is their specificity in DNA cleavage. Different types of endonucleases exhibit varying degrees of specificity, allowing them to target specific DNA sequences. This specificity is crucial for the precise regulation of DNA processes in cells.
Endonucleases can recognize specific DNA sequences through various mechanisms. Some endonucleases recognize palindromic sequences, which are sequences that read the same forward and backward on complementary DNA strands. These endonucleases bind to the palindromic sequence and cleave the DNA at a specific site within that sequence.
Other endonucleases exhibit sequence-specific recognition, where they recognize a specific sequence of nucleotides without requiring palindromic symmetry. These endonucleases have specific binding sites that match the target DNA sequence, allowing them to bind and cleave the DNA at the desired location.
The specificity of endonucleases in DNA cleavage is crucial for their role in various biological processes. By precisely targeting specific DNA sequences, endonucleases can initiate DNA repair, remove damaged DNA, or facilitate DNA recombination.
Exonuclease Enzyme Examples
Exonucleases are a class of enzymes that play a crucial role in DNA and RNA metabolism. These enzymes are responsible for removing nucleotides from the ends of DNA or RNA molecules. By doing so, they contribute to various cellular processes such as DNA repair, DNA replication, and RNA degradation. Let’s explore some examples of exonucleases and their functions.
1. DNA Polymerase I
One well-known example of an exonuclease is DNA Polymerase I, which is found in bacteria. This enzyme has both polymerase and exonuclease activities. During DNA replication, DNA Polymerase I synthesizes new DNA strands by adding nucleotides to the growing chain. However, it also possesses a 5′ to 3′ exonuclease activity that allows it to remove nucleotides from the DNA molecule. This exonuclease activity is particularly important for DNA repair processes, as it enables the removal of damaged or mismatched nucleotides.
2. Exonuclease 1 (EXO1)
Exonuclease 1, also known as EXO1, is a highly conserved enzyme found in eukaryotes. It plays a crucial role in DNA repair and recombination. EXO1 is primarily involved in the resection of DNA ends during homologous recombination and DNA double-strand break repair. By removing nucleotides from the DNA ends, EXO1 generates single-stranded DNA regions that are essential for the repair process. Additionally, EXO1 has been implicated in DNA mismatch repair and the maintenance of genomic stability.
3. XRN Exonucleases
XRN exonucleases are a family of enzymes found in both prokaryotes and eukaryotes. These enzymes are involved in RNA degradation and play a crucial role in regulating gene expression. XRN exonucleases degrade RNA molecules in a 5′ to 3′ direction, removing nucleotides one by one from the RNA molecule’s end. In eukaryotes, XRN exonucleases are responsible for degrading mRNA molecules that are no longer needed or have become damaged. This process helps control gene expression and ensures the removal of aberrant RNA molecules.
4. RNase II
RNase II is an exonuclease found in bacteria that is involved in RNA degradation. It plays a crucial role in maintaining RNA homeostasis by degrading unwanted or damaged RNA molecules. RNase II exhibits 3′ to 5′ exonuclease activity, meaning it removes nucleotides from the 3′ end of RNA molecules. This activity allows RNase II to degrade RNA molecules in a process known as exonucleolytic degradation. By removing nucleotides one by one, RNase II contributes to the turnover of RNA molecules and helps regulate gene expression.
Restriction Endonuclease Quizlet
Restriction endonucleases, also known as restriction enzymes, are a class of enzymes that play a crucial role in molecular biology. These enzymes are found in bacteria and archaea, where they act as a defense mechanism against foreign DNA, such as viral DNA. In this section, we will explore the fascinating world of restriction endonucleases and their significance in genetic research.
What are Restriction Endonucleases?
Restriction endonucleases are enzymes that can recognize specific DNA sequences and cleave the DNA at those sites. They are named “restriction” enzymes because they restrict the growth of bacteriophages, which are viruses that infect bacteria. These enzymes are essential tools in genetic engineering and molecular biology, as they allow scientists to cut DNA molecules at precise locations.
How do Restriction Endonucleases Work?
Restriction endonucleases recognize and bind to specific DNA sequences, known as recognition sites or restriction sites. These recognition sites are usually palindromic, meaning they read the same forward and backward on both DNA strands. Once the enzyme binds to the recognition site, it cleaves the DNA, breaking the phosphodiester bonds and generating fragments.
Types of Restriction Endonucleases
There are three main types of restriction endonucleases: Type I, Type II, and Type III. Each type has different characteristics and mechanisms of action.
-
Type I Restriction Endonucleases: These enzymes recognize specific DNA sequences but cleave the DNA at random sites, often far away from the recognition site.
-
Type II Restriction Endonucleases: Type II enzymes are the most commonly used restriction endonucleases in research. They recognize specific DNA sequences and cleave the DNA at or near the recognition site. Type II enzymes are further classified into four subtypes: IIS, IIT, IIE, and IIG.
-
Type III Restriction Endonucleases: Type III enzymes also recognize specific DNA sequences, but their cleavage sites are variable and depend on the distance between the recognition site and another specific sequence called the “res” site.
Examples of Restriction Endonucleases
There are numerous examples of restriction endonucleases, each with its own recognition sequence and cleavage pattern. Some well-known examples include:
-
EcoRI: This enzyme recognizes the DNA sequence GAATTC and cleaves between the G and the A.
-
HindIII: HindIII recognizes the DNA sequence AAGCTT and cleaves between the A and the G.
-
BamHI: BamHI recognizes the DNA sequence GGATCC and cleaves between the G and the A.
These examples represent just a small fraction of the many restriction endonucleases that have been discovered and characterized.
Applications of Restriction Endonucleases
Restriction endonucleases have numerous applications in genetic research and biotechnology. Some of the key applications include:
-
DNA Cloning: Restriction endonucleases are used to cut DNA molecules at specific sites, allowing scientists to insert or remove specific genes or DNA fragments.
-
DNA Analysis: These enzymes are used in techniques such as restriction fragment length polymorphism (RFLP) analysis, which helps identify genetic variations and mutations.
-
Gene Mapping: By using restriction endonucleases to cut DNA at specific sites, scientists can create a map of the locations of genes on a chromosome.
-
DNA Sequencing: Restriction endonucleases play a crucial role in DNA sequencing methods, such as the Sanger sequencing method.
What Do Endonucleases Do?
Endonucleases are enzymes that play a crucial role in various biological processes involving DNA. They are responsible for cleaving the phosphodiester bonds within the DNA molecule, leading to the fragmentation of the DNA strand. This ability to cut DNA at specific sites makes endonucleases essential for DNA repair, recombination, and other cellular functions.
Role in DNA Fragmentation
One of the primary functions of endonucleases is to fragment DNA. These enzymes can recognize specific DNA sequences and cleave the phosphodiester bonds within the DNA backbone at those sites. This fragmentation is a critical step in many molecular biology techniques and experiments, such as DNA sequencing and genetic engineering.
Endonucleases are often used to generate DNA fragments of a desired size for further analysis or manipulation. For example, in DNA sequencing, endonucleases are employed to break the DNA into smaller fragments that can be sequenced individually. This fragmentation allows scientists to obtain a complete sequence of the DNA molecule.
Involvement in DNA Repair and Recombination
Endonucleases also play a vital role in DNA repair and recombination processes. When DNA is damaged, endonucleases are responsible for recognizing and cleaving the damaged DNA strand. This cleavage initiates the repair process by removing the damaged section of DNA.
In DNA recombination, endonucleases are involved in the exchange of genetic material between two DNA molecules. These enzymes recognize specific DNA sequences known as recombination sites and cleave the DNA at those sites. This cleavage allows for the exchange and rearrangement of genetic material, leading to genetic diversity and the creation of new combinations of genes.
Different types of endonucleases are involved in specific DNA repair and recombination pathways. For example, the enzyme called Flap endonuclease 1 (FEN1) is involved in the repair of DNA damage caused by replication errors, while the enzyme called Holliday junction endonuclease is involved in DNA recombination during meiosis.
Overall, endonucleases are essential enzymes that play a crucial role in DNA fragmentation, repair, and recombination. Their ability to cleave DNA at specific sites allows for precise manipulation of DNA molecules in various biological processes. By understanding the functions and mechanisms of endonucleases, scientists can gain valuable insights into the complex world of DNA and its role in life processes.
Where Do Restriction Enzymes Cut DNA
Restriction enzymes, also known as endonucleases, are a class of enzymes that play a crucial role in molecular biology. These enzymes have the remarkable ability to recognize specific DNA sequences and cleave the DNA at precise locations. This section will explore the recognition sequences of restriction enzymes and the cleavage sites in DNA.
Recognition Sequences of Restriction Enzymes
Each restriction enzyme has a specific recognition sequence, which is a short DNA sequence that the enzyme identifies and binds to. These recognition sequences are typically palindromic, meaning they read the same forward and backward on the two DNA strands. For example, the recognition sequence for the popular restriction enzyme EcoRI is 5′-GAATTC-3′. This sequence is palindromic, as it reads the same on both strands: 5′-GAATTC-3′ on one strand and 3′-CTTAAG-5′ on the complementary strand.
Different restriction enzymes recognize different recognition sequences. This diversity allows scientists to selectively target specific DNA sequences for manipulation. By using different restriction enzymes with distinct recognition sequences, researchers can create precise cuts in DNA at desired locations.
Cleavage Sites in DNA
Once a restriction enzyme recognizes its specific recognition sequence, it cleaves the DNA at a specific site. The cleavage site is typically within or near the recognition sequence. The position of the cleavage site varies depending on the specific restriction enzyme.
Restriction enzymes can create two types of cuts in DNA: blunt ends and sticky ends. Blunt ends are created when the enzyme cuts the DNA straight across both strands, resulting in a clean break. Sticky ends, on the other hand, are created when the enzyme cuts the DNA in a staggered manner, leaving short, single-stranded overhangs at the ends. These overhangs can then bind with complementary overhangs from other DNA molecules, allowing for the creation of recombinant DNA molecules.
The specific cleavage site of a restriction enzyme depends on its recognition sequence. For example, EcoRI cleaves the DNA at the specific site between the G and the A in its recognition sequence, resulting in sticky ends with a 5′ overhang of AATT. Other restriction enzymes have different cleavage sites within their recognition sequences, leading to the generation of unique DNA fragments with specific ends.
When Are Restriction Enzymes Used
Restriction enzymes, also known as endonucleases, are essential tools in molecular biology research and DNA manipulation techniques. These enzymes play a crucial role in various applications, making them indispensable in the field. Let’s explore some of the key areas where restriction enzymes find their utility.
Applications in Molecular Biology Research
Restriction enzymes are extensively used in molecular biology research to study DNA structure, function, and genetic information. Here are a few notable applications:
-
DNA Sequencing: Restriction enzymes help in DNA sequencing by cutting DNA at specific recognition sites. This allows scientists to analyze the order of nucleotides in a DNA molecule, providing valuable insights into genetic information.
-
Gene Cloning: Restriction enzymes are vital in gene cloning, a technique used to create multiple copies of a specific DNA fragment. These enzymes precisely cleave DNA at specific sites, enabling the insertion of the desired DNA fragment into a vector for replication.
-
Genetic Engineering: Restriction enzymes are instrumental in genetic engineering, where foreign DNA is introduced into an organism’s genome. By cutting DNA at specific sites, restriction enzymes facilitate the insertion of foreign DNA into the host organism, allowing the production of desired proteins or modification of genetic traits.
-
DNA Fragment Analysis: Restriction enzymes are used to analyze DNA fragments in techniques such as restriction fragment length polymorphism (RFLP) analysis. By cutting DNA at specific sites, these enzymes generate unique fragment patterns that can be used to identify genetic variations or analyze DNA samples.
Use in DNA Manipulation Techniques
Restriction enzymes are also widely employed in various DNA manipulation techniques, enabling scientists to modify and study DNA molecules. Some notable uses include:
-
DNA Digestion: Restriction enzymes are used to digest DNA molecules into smaller fragments. This process is crucial for various downstream applications, such as DNA sequencing, gene mapping, and DNA fragment analysis.
-
DNA Ligation: After DNA digestion, restriction enzymes can be used to ligate or join DNA fragments together. This process is essential in gene cloning, where DNA fragments are inserted into vectors for replication.
-
Site-Directed Mutagenesis: Restriction enzymes play a vital role in site-directed mutagenesis, a technique used to introduce specific mutations into DNA sequences. By cutting DNA at specific sites, restriction enzymes enable the replacement of nucleotides, allowing scientists to study the effects of specific mutations.
-
DNA Labeling: Restriction enzymes can be used to incorporate labeled nucleotides into DNA molecules. This technique is valuable in labeling specific DNA fragments for visualization or tracking purposes.
Why Do Restriction Endonucleases Cut Certain DNA Segments
Restriction endonucleases, also known as restriction enzymes, are a class of enzymes that play a crucial role in DNA manipulation and genetic engineering. These enzymes are responsible for cutting DNA at specific recognition sites, which are usually palindromic sequences. This section will explore the reasons behind why restriction endonucleases cut certain DNA segments.
Specificity of restriction enzymes
One of the remarkable features of restriction endonucleases is their specificity. Each restriction enzyme recognizes a specific DNA sequence and cleaves it at a particular site. This specificity is essential for their function in DNA manipulation. The recognition sites are typically short sequences, ranging from four to eight base pairs in length.
Restriction enzymes are named after the bacteria from which they were originally isolated. For example, EcoRI is derived from Escherichia coli strain RY13, and HindIII is derived from Haemophilus influenzae strain Rd. The specificity of restriction enzymes allows scientists to precisely target and manipulate specific regions of DNA.
Role in bacterial defense mechanisms
Restriction endonucleases are not only valuable tools in genetic engineering but also play a crucial role in bacterial defense mechanisms. Bacteria use restriction enzymes as a defense mechanism against invading foreign DNA, such as bacteriophages (viruses that infect bacteria).
When a bacteriophage injects its DNA into a bacterial cell, the restriction enzymes recognize the foreign DNA and cleave it at specific sites. This cleavage prevents the bacteriophage from replicating and effectively neutralizes the threat. The bacterial cell’s own DNA remains unharmed due to the presence of methyl groups added to specific bases, which protect it from restriction enzyme cleavage.
The ability of restriction enzymes to cut foreign DNA while leaving the host DNA intact is a critical defense mechanism for bacteria. This mechanism helps bacteria survive and protect their genetic material from potential harm.
What Is Endonuclease Enzyme?
Endonucleases are a class of enzymes that play a crucial role in DNA and RNA metabolism. These enzymes are responsible for cleaving the phosphodiester bonds within a nucleic acid chain. The term “endonuclease” is derived from the Greek words “endon,” meaning “within,” and “nuclease,” referring to an enzyme that breaks down nucleic acids.
Definition and Function of Endonucleases
Endonucleases are enzymes that cleave the phosphodiester bonds within a nucleic acid chain, resulting in the fragmentation of the chain. Unlike exonucleases, which cleave nucleic acids from the ends, endonucleases cleave within the chain itself. This ability to cleave internally is what sets endonucleases apart and makes them essential for various biological processes.
The primary function of endonucleases is to maintain the integrity and stability of DNA and RNA molecules. They are involved in DNA repair, replication, recombination, and gene expression. By cleaving the nucleic acid chain at specific sites, endonucleases facilitate the removal of damaged or mismatched bases, the excision of introns during RNA splicing, and the processing of precursor RNA molecules into mature forms.
Types of Endonucleases
There are several types of endonucleases, each with its own unique characteristics and functions. Some of the most well-known types include:
-
Restriction Endonucleases: These enzymes are commonly found in bacteria and are part of the bacterial defense system against foreign DNA. Restriction endonucleases recognize specific DNA sequences and cleave the DNA at or near these recognition sites. They are widely used in molecular biology research for DNA manipulation and genetic engineering.
-
Homing Endonucleases: Also known as meganucleases, homing endonucleases are enzymes that can recognize and cleave specific DNA sequences. They are often involved in the mobility of genetic elements, such as introns and inteins, within genomes.
-
DNA Repair Endonucleases: These enzymes are crucial for maintaining the integrity of the genome by repairing DNA damage. They recognize and cleave DNA at sites of damage, allowing for the removal and replacement of damaged DNA segments.
-
RNA Splicing Endonucleases: These enzymes are involved in the processing of precursor RNA molecules during RNA splicing. They cleave the RNA chain at specific sites, allowing for the removal of introns and the joining of exons to form a mature RNA molecule.
Endonuclease Examples
Here are a few examples of well-known endonucleases:
-
EcoRI: EcoRI is a restriction endonuclease derived from the bacterium Escherichia coli. It recognizes the DNA sequence GAATTC and cleaves the DNA between the G and the A.
-
Cas9: Cas9 is an RNA-guided endonuclease derived from the CRISPR-Cas9 system found in bacteria. It is widely used in genome editing and gene therapy research due to its ability to cleave DNA at specific locations guided by a complementary RNA molecule.
-
Flap Endonuclease 1 (FEN1): FEN1 is an endonuclease involved in DNA replication and repair. It cleaves the DNA flap structures that form during DNA synthesis and recombination, ensuring the proper processing and maintenance of DNA.
Endonucleases are essential enzymes that play a vital role in DNA and RNA metabolism. They are involved in various biological processes, including DNA repair, replication, recombination, and gene expression. Understanding the structure, mechanism, and specificity of endonucleases has paved the way for advancements in molecular biology research and applications such as genetic engineering and gene therapy.
How Do Restriction Endonucleases Work?
Restriction endonucleases, also known as restriction enzymes, are a class of enzymes that play a crucial role in DNA cleavage. These enzymes are found in bacteria and are responsible for protecting the bacterial cell from foreign DNA, such as that from viruses. In this section, we will explore the mechanism of action of restriction endonucleases and the role of cofactors and recognition sites in their function.
Mechanism of Action in DNA Cleavage
The mechanism of action of restriction endonucleases involves the recognition and cleavage of specific DNA sequences. These enzymes are highly specific and can recognize and bind to particular DNA sequences, known as recognition sites. Once bound to the recognition site, the restriction endonuclease cuts the DNA molecule at specific points, resulting in the cleavage of the DNA.
Restriction endonucleases can cleave DNA in two different ways: blunt-ended cleavage and sticky-ended cleavage. In blunt-ended cleavage, the enzyme cuts the DNA molecule at the same position on both strands, resulting in blunt ends. On the other hand, in sticky-ended cleavage, the enzyme cuts the DNA molecule at different positions on the two strands, creating single-stranded overhangs, or sticky ends.
The cleavage activity of restriction endonucleases is essential for their role in DNA manipulation and genetic engineering. By cutting DNA at specific sites, these enzymes allow scientists to insert or remove specific DNA fragments, facilitating the study of gene function and the development of new technologies.
Role of Cofactors and Recognition Sites
Cofactors play a crucial role in the function of restriction endonucleases. These cofactors are often metal ions, such as magnesium or calcium, which are required for the enzyme to function properly. Cofactors help in stabilizing the enzyme-substrate complex and facilitating the cleavage of the DNA molecule.
Recognition sites are specific DNA sequences that are recognized and bound by restriction endonucleases. These recognition sites are usually palindromic, meaning they read the same on both strands when read in the 5′ to 3′ direction. For example, the recognition site for the restriction endonuclease EcoRI is 5′-GAATTC-3′, which is palindromic.
The specificity of restriction endonucleases is determined by the sequence of the recognition site. Different restriction endonucleases recognize and cleave different DNA sequences. This specificity allows scientists to selectively cleave DNA at specific sites, enabling precise manipulation of DNA molecules.
Why Are Restriction Enzymes Also Called Restriction Endonucleases
Restriction enzymes, also known as restriction endonucleases, are a class of enzymes that play a crucial role in molecular biology research. These enzymes are named “restriction endonucleases” due to their ability to cleave DNA at specific recognition sites, which are often palindromic sequences. Let’s delve deeper into why these enzymes are referred to by both names.
The Role of Restriction Enzymes
Restriction enzymes are naturally occurring proteins that bacteria produce as a defense mechanism against invading viruses, known as bacteriophages. These enzymes recognize specific DNA sequences and cleave the DNA at or near these sites, thereby protecting the bacterial cell from viral infection.
The Cleavage Mechanism
Restriction enzymes cleave DNA by breaking the phosphodiester bonds that hold the nucleotides together. This cleavage can occur in two ways: endonucleolytic cleavage and exonucleolytic cleavage. Endonucleolytic cleavage refers to the breaking of the DNA strand within the recognition site, while exonucleolytic cleavage involves the removal of nucleotides from the ends of the DNA molecule.
Recognition and Specificity
One of the remarkable features of restriction enzymes is their ability to recognize and bind to specific DNA sequences. These recognition sequences are typically palindromic, meaning they read the same forward and backward on complementary strands. For example, the recognition sequence for the restriction enzyme EcoRI is 5′-GAATTC-3′, which is palindromic.
Types of Restriction Enzymes
There are three main types of restriction enzymes: Type I, Type II, and Type III. Type II restriction enzymes are the most commonly used in molecular biology research. They recognize specific DNA sequences and cleave the DNA at or near these sites. Type I and Type III restriction enzymes, on the other hand, cleave DNA at sites that are distant from the recognition sequence.
Examples of Restriction Enzymes
There are numerous examples of restriction enzymes, each with its own recognition sequence and cleavage pattern. Some well-known examples include EcoRI, HindIII, BamHI, and PstI. These enzymes have been extensively studied and are widely used in molecular biology techniques such as DNA cloning, DNA sequencing, and genetic engineering.
Applications in Molecular Biology
The discovery and characterization of restriction enzymes revolutionized the field of molecular biology. These enzymes have enabled scientists to manipulate DNA in ways that were previously unimaginable. By using restriction enzymes to cleave DNA at specific sites, researchers can insert or remove specific DNA fragments, allowing them to study gene function, create recombinant DNA molecules, and develop new therapeutic strategies.
When Do Restriction Enzymes Cut
Restriction enzymes, also known as endonucleases, are essential tools in molecular biology. These enzymes play a crucial role in DNA manipulation by cutting DNA molecules at specific recognition sites. Understanding when restriction enzymes cut is fundamental to their successful application in various molecular biology techniques, such as DNA cloning and genetic engineering.
Recognition Sites
Restriction enzymes recognize specific DNA sequences, known as recognition sites, and cut the DNA at or near these sites. The recognition sites are typically palindromic, meaning they read the same on both strands when oriented in the 5′ to 3′ direction. For example, the recognition site for the restriction enzyme EcoRI is 5′-GAATTC-3′, which is the same sequence on both strands when read in the 5′ to 3′ direction.
Cleavage Patterns
Restriction enzymes can cut DNA in different ways, resulting in different cleavage patterns. The two most common types of cleavage patterns are blunt-ended and sticky-ended.
-
Blunt-ended cleavage: Some restriction enzymes cut both DNA strands at the recognition site, resulting in blunt ends. Blunt ends are straight cuts that do not leave any overhanging nucleotides. For example, the restriction enzyme EcoRV cuts DNA at the recognition site 5′-GATATC-3‘, generating blunt ends.
-
Sticky-ended cleavage: Other restriction enzymes cut the DNA strands at the recognition site, but in an offset manner, resulting in sticky ends. Sticky ends have short, single-stranded overhangs that can base pair with complementary sequences. These overhangs can be useful for DNA ligation and cloning. For instance, the restriction enzyme EcoRI cuts DNA at the recognition site 5′-GAATTC-3‘, generating sticky ends with a 5’ overhang of “AATT” on one strand and a 3′ overhang of “TTAA” on the other strand.
Factors Affecting Enzyme Activity
Several factors can influence the activity of restriction enzymes and determine when they cut DNA effectively:
-
Temperature: Different restriction enzymes have different optimal temperatures for activity. Most restriction enzymes work best at moderate temperatures, typically between 37°C and 65°C.
-
pH: The pH of the reaction buffer can affect the activity of restriction enzymes. Most restriction enzymes function optimally at a slightly acidic to neutral pH range, around pH 7.
-
Salt concentration: The salt concentration in the reaction buffer can impact the activity of restriction enzymes. Most restriction enzymes require a certain salt concentration, usually provided by the addition of magnesium ions (Mg2+), for optimal activity.
-
DNA methylation: Some restriction enzymes are sensitive to DNA methylation, a chemical modification of DNA. Methylation can prevent restriction enzymes from cutting at their recognition sites. This sensitivity to DNA methylation is often utilized as a defense mechanism by bacteria to protect their own DNA from being cleaved by their own restriction enzymes.
Is an endonuclease enzyme an example of a protein?
Yes, an endonuclease enzyme is indeed an example of a protein. Enzymes, including endonucleases, are a specific class of proteins that act as catalysts in various biological reactions. They play a crucial role in breaking down or building up chemical bonds within other molecules, such as DNA. Enzymes’ role as a protein is central to their function, as their structure and composition determine their specific catalytic activity. To learn more about the role of enzymes as proteins, you can visit Enzyme’s role as a protein.
Frequently Asked Questions
1. What is an endonuclease enzyme?
An endonuclease enzyme is a type of enzyme that cleaves DNA or RNA at specific sites within the nucleotide sequence.
2. Where are endonucleases found?
Endonucleases are found in various organisms, including bacteria, archaea, and eukaryotes.
3. How do endonucleases cut DNA?
Endonucleases cut DNA by breaking the phosphodiester bonds within the DNA molecule, resulting in the cleavage of the DNA strand.
4. What do endonucleases do?
Endonucleases play a crucial role in DNA repair, recombination, replication, and gene regulation by cleaving DNA at specific sites.
5. What is the function of endonucleases?
The function of endonucleases is to cleave DNA or RNA at specific sites, allowing for various biological processes such as DNA repair and gene expression.
6. What are some examples of endonuclease enzymes?
Examples of endonuclease enzymes include restriction endonucleases, which are commonly used in molecular biology research, and exonucleases, which degrade DNA or RNA from the ends.
7. How do restriction endonucleases work?
Restriction endonucleases recognize specific DNA sequences and cleave the DNA at or near these sequences, resulting in the generation of DNA fragments with sticky or blunt ends.
8. Why do restriction endonucleases cut certain DNA segments?
Restriction endonucleases cut certain DNA segments because they recognize specific DNA sequences, known as restriction sites, which are typically palindromic in nature.
9. When are restriction enzymes used?
Restriction enzymes are used in various molecular biology techniques, such as DNA cloning, DNA fingerprinting, and gene expression analysis.
10. When were restriction enzymes first isolated?
Restriction enzymes were first isolated in 1970 by Hamilton O. Smith and colleagues, leading to significant advancements in the field of molecular biology.
Also Read: