Nuclear fusion in the sun is a complex process where hydrogen atoms are converted into helium, releasing massive amounts of energy in the process. This reaction takes place in the sun’s core, where the temperature, density, and pressure are extremely high, allowing the nuclei to overcome their electrical repulsion and fuse. Understanding the intricate details of this process is crucial for developing fusion as a viable energy source for the future.
The Fundamentals of Nuclear Fusion
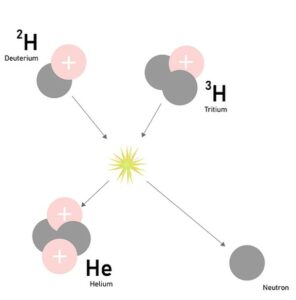
Nuclear fusion is the process of combining two light atomic nuclei to form a single, heavier nucleus. This reaction releases a significant amount of energy, making it a promising source of clean and renewable energy. In the sun, the fusion process involves the isotopes of hydrogen, specifically deuterium (2H) and tritium (3H), which combine to form helium (4He).
The fusion reaction can be represented by the following equation:
2H + 3H → 4He + n + 17.6 MeV
Where:
– 2H is deuterium
– 3H is tritium
– 4He is helium
– n is a neutron
– 17.6 MeV is the energy released per fusion event
The high temperature, density, and pressure in the sun’s core are essential for this fusion process to occur. The temperature in the sun’s core is approximately 15 million degrees Celsius, which provides the necessary energy for the nuclei to overcome their mutual electrical repulsion and come close enough for the strong nuclear force to take over and fuse them together.
The Conditions for Fusion in the Sun
The sun’s core is a unique environment that creates the ideal conditions for nuclear fusion to occur. Let’s explore the key factors that contribute to this process:
Temperature
The temperature in the sun’s core is around 15 million degrees Celsius, which is hot enough to ionize the atoms, creating a plasma state. This high temperature provides the necessary kinetic energy for the nuclei to overcome the Coulomb barrier and fuse together.
Density
The density of the sun’s core is approximately 150 times the density of water, or about 150 g/cm³. This high density increases the probability of collisions between the nuclei, which is essential for the fusion process.
Pressure
The pressure in the sun’s core is approximately 250 billion times the atmospheric pressure at sea level, or about 2.5 × 1011 Pa. This immense pressure, generated by the sun’s gravity, helps to confine the plasma and maintain the high temperature and density required for fusion.
Confinement
The high pressure and density in the sun’s core help to confine the plasma, increasing the chances of collisions between the nuclei. This confinement is crucial for the fusion process, as it increases the probability of the nuclei coming close enough to overcome the Coulomb barrier and fuse.
The Fusion Reaction Cycle
The fusion process in the sun’s core is a continuous cycle, where hydrogen atoms are converted into helium, releasing energy in the process. This cycle can be divided into the following steps:
-
Proton-Proton Chain Reaction: The fusion process begins with the proton-proton chain reaction, where two hydrogen nuclei (protons) fuse to form a deuterium nucleus (2H) and a positron (e+).
-
Deuterium Fusion: The deuterium nucleus then fuses with another hydrogen nucleus to form a tritium nucleus (3H) and a gamma ray (γ).
-
Tritium Fusion: The tritium nucleus then fuses with another deuterium nucleus to form a helium nucleus (4He) and a neutron (n), releasing 17.6 MeV of energy.
-
Energy Radiation: The energy released by the fusion process is then radiated outward, taking about 100,000 years to reach the sun’s surface and then about 8 minutes and 20 seconds to reach Earth.
This continuous cycle of fusion reactions in the sun’s core is responsible for the energy that sustains life on Earth, providing heat and light.
The Energy Output of the Sun’s Fusion Process
The sun’s fusion process converts about 4 million tons of matter into energy every second, which is an incredibly large amount of energy. This energy is released in the form of heat and light, which then radiates outward from the sun’s core.
The energy output of the sun’s fusion process can be calculated using the following formula:
E = mc²
Where:
– E is the energy released
– m is the mass of the matter converted
– c is the speed of light
Substituting the values, we get:
E = (4 × 106 kg/s) × (3 × 108 m/s)²
E = 3.6 × 1026 W
This means that the sun’s fusion process generates an astounding 3.6 × 10^26 watts of power, which is then radiated outward and reaches Earth in the form of heat and light.
Challenges in Achieving Fusion on Earth
While the sun’s fusion process is a continuous and efficient source of energy, replicating these conditions on Earth has proven to be a significant challenge. The main challenges in achieving fusion on Earth include:
-
Achieving Sufficient Temperature: The temperature required for fusion to occur on Earth is around 100 million degrees Celsius, which is significantly higher than the temperature in the sun’s core.
-
Maintaining Plasma Confinement: Maintaining the high-density plasma required for fusion is a major challenge, as the plasma tends to be unstable and difficult to confine.
-
Overcoming Material Limitations: The materials used in fusion reactors must be able to withstand the extreme temperatures and pressures involved in the fusion process, which poses significant engineering challenges.
-
Achieving Net Energy Gain: Ensuring that the energy output of the fusion process is greater than the energy required to initiate and sustain the reaction is a critical challenge.
Despite these challenges, significant progress has been made in the development of fusion reactors, and the potential for fusion as a clean and sustainable energy source remains a promising avenue of research.
Conclusion
Nuclear fusion in the sun is a complex and fascinating process that involves the conversion of hydrogen into helium, releasing massive amounts of energy in the process. The high temperature, density, and pressure in the sun’s core create the ideal conditions for this fusion process to occur, and the energy released is essential for sustaining life on Earth.
Understanding the intricacies of the sun’s fusion process can provide valuable insights into the development of fusion as a viable energy source for the future. While the challenges in achieving fusion on Earth are significant, the potential benefits of this clean and sustainable energy source make it a worthy pursuit for scientists and engineers around the world.