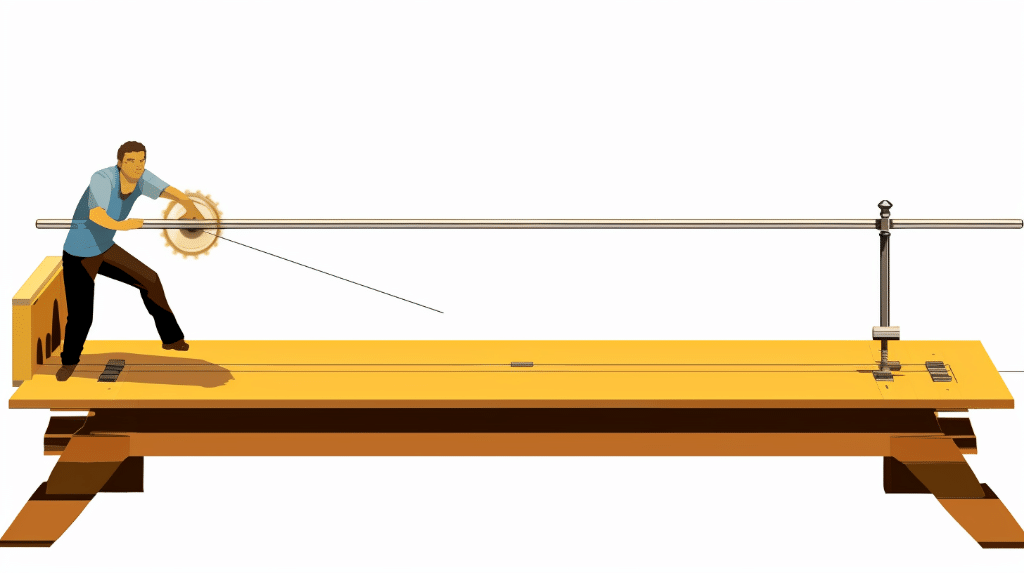
Torque is a fundamental concept in physics that describes the rotational force applied to an object. It plays a crucial role in understanding rotational motion and is essential for various applications, such as engineering, mechanics, and even sports. In this blog post, we will explore how to find torque with mass and distance, providing you with a clear understanding of the underlying principles and practical calculations involved.
How to Calculate Torque with Mass and Distance
The Torque Equation
To calculate torque, we use the equation:
![]()
In this equation, the force refers to the force applied to the object, and the distance is the perpendicular distance between the axis of rotation and the point where the force is applied. The unit of torque is typically expressed in Newton-meters (Nm) or foot-pounds (ft-lb), depending on the unit system used.
Steps to Calculate Torque Given Mass and Distance
To calculate torque using mass and distance, you need to follow these steps:
- Identify the force applied to the object. This force can be due to various factors, such as gravity, friction, or an external force applied intentionally.
- Determine the distance from the axis of rotation to the point where the force is applied. This distance should be measured perpendicular to the line of action of the force.
- Multiply the force by the distance to calculate the torque.
Let’s walk through an example to illustrate this process.
Worked Out Example: Calculating Torque with Mass and Distance
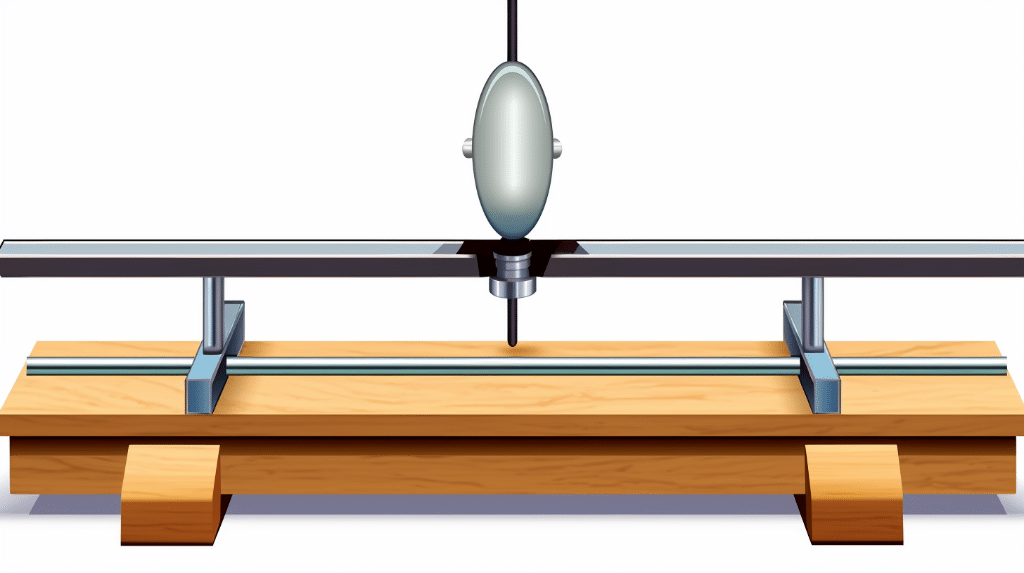
Suppose we have a wooden beam of mass 10 kg, and we apply a force of 20 N at a distance of 2 meters from the axis of rotation. To find the torque, we can use the equation mentioned earlier:
![]()
Substituting the values, we get:
![]()
Thus, the torque applied to the wooden beam is 40 Nm.
Advanced Concepts in Finding Torque
Finding Torque for Angled Forces

In some cases, the force may not act perpendicular to the line connecting the point of application to the axis of rotation. In such situations, we can find the torque by considering the perpendicular component of the force.
To calculate the torque when the force is at an angle, we use the following modified equation:
![]()
Where:
– ![]() is the angle between the force vector and the line connecting the point of application to the axis of rotation.
is the angle between the force vector and the line connecting the point of application to the axis of rotation.
How to Find Torque without Mass
While mass is commonly used to calculate torque, it is essential to note that torque can also be determined without explicitly considering the mass of the object. In such cases, we rely on the concept of moment of inertia.
The moment of inertia ![]() ) of an object is a measure of its resistance to rotational motion. It depends on both the mass distribution and the axis of rotation. The torque can be calculated using the following equation:
) of an object is a measure of its resistance to rotational motion. It depends on both the mass distribution and the axis of rotation. The torque can be calculated using the following equation:
![]()
Where:
– ![]() is a property of the object determined by its mass distribution and shape,
is a property of the object determined by its mass distribution and shape,
– ![]() refers to the rate of change of angular velocity.
refers to the rate of change of angular velocity.
How to Find Torque without Force
Similarly, torque can also be determined without explicitly knowing the force applied to the object. This is possible when the angular acceleration is known. In such cases, we can use the following equation to calculate torque:
![]()
This equation is derived from Newton’s second law for rotational motion, which states that the torque on an object is equal to the product of moment of inertia and angular acceleration.
Finding the Magnitude of the Torque
In certain scenarios, it may be necessary to find the magnitude of the torque acting on an object without considering its direction. To do so, we can use the following equation:
![]()
The lever arm length is the perpendicular distance between the axis of rotation and the line of action of the force.
Practical Applications of Torque Calculations
How to Find Torque on a Wheel
One practical application of torque calculations is determining the torque exerted on a wheel. This is particularly important in the automotive industry, where torque is used to measure the force that drives a vehicle’s wheels. By accurately calculating the torque, engineers can design more efficient and powerful vehicles.
How to Find the Mass of a Ruler Using Torque
Torque can also be used to find the mass of an object with a known distance and force applied. For example, consider a ruler balanced on a pivot point. By applying a known force at a measured distance from the pivot, we can calculate the mass of the ruler using the torque equation.
How to Find Center of Mass with Torque
Torque calculations can be used to determine the center of mass of an object. By applying a force to the object at different distances from the axis of rotation and measuring the resulting torque, we can find the position of the center of mass. This information is crucial in various fields, including engineering, physics, and biomechanics.
By understanding how to find torque with mass and distance, you can apply this knowledge to solve various real-world problems involving rotational motion.
Numerical Problems on how to find torque with mass
- A disk with a mass of 2 kg is rotating with an angular velocity of 3 radians per second. Calculate the torque exerted on the disk.
Solution:
Given:
Mass of the disk, ![]()
Angular velocity, ![]()
The formula to calculate torque is given by:![]()
Where:![]() is the moment of inertia of the disk
is the moment of inertia of the disk![]() is the angular acceleration of the disk
is the angular acceleration of the disk
To find the moment of inertia, we can use the formula:![]()
Where:![]() is the radius of the disk
is the radius of the disk
Assuming the disk has a radius of 0.5 meters, we can calculate the moment of inertia:![]()
Since the angular acceleration is zero (as there is no change in angular velocity), the torque exerted on the disk is also zero.
Therefore, the torque exerted on the disk is 0 Nm.
![]() : # (This is a comment in Markdown format, the LaTeX expression should not be rendered by the markdown renderer.)
: # (This is a comment in Markdown format, the LaTeX expression should not be rendered by the markdown renderer.)
- A uniform rod of length 1.5 meters and mass 4 kg is pivoted at one end and is at rest. A force of 10 N is applied perpendicular to the rod at a distance of 0.5 meters from the pivot point. Find the torque exerted on the rod by the force.
Solution:
Given:
Length of the rod, ![]()
Mass of the rod, ![]()
Force applied, ![]()
Distance from the pivot point, ![]()
The formula to calculate torque is given by:![]()
Substituting the given values:![]()
Therefore, the torque exerted on the rod by the force is 5 Nm.
- A wheel with a moment of inertia of 0.2 kg·m² is rotating with an angular velocity of 4 radians per second. Calculate the torque required to stop the wheel in 2 seconds.
Solution:
Given:
Moment of inertia of the wheel, ![]()
Angular velocity, ![]()
Time, ![]()
The formula to calculate torque is given by:![]()
Where:![]() is the angular acceleration of the wheel
is the angular acceleration of the wheel
Since we want to stop the wheel, the final angular velocity will be 0 rad/s. We can find the angular acceleration using the formula:![]()
Substituting the given values:![]()
Solving for ![]() :
:![]()
Substituting the values of ![]() and
and ![]() in the torque formula:
in the torque formula:![]()
Therefore, the torque required to stop the wheel in 2 seconds is -0.4 Nm.
Also Read:
- How to find binding energy from mass defect
- Law of conservation of mass
- How to find potential energy with height and mass
- How to calculate friction force without mass
- Biomass energy
- How to find kinetic energy with speed and mass
- How to find potential energy without mass
- How to find normal force with mass
- How to find mass in centripetal force
- How to find kinetic energy with mass and height