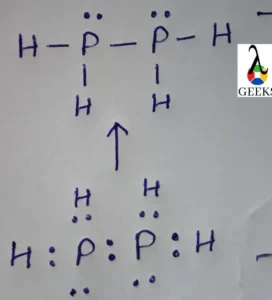
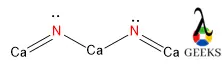
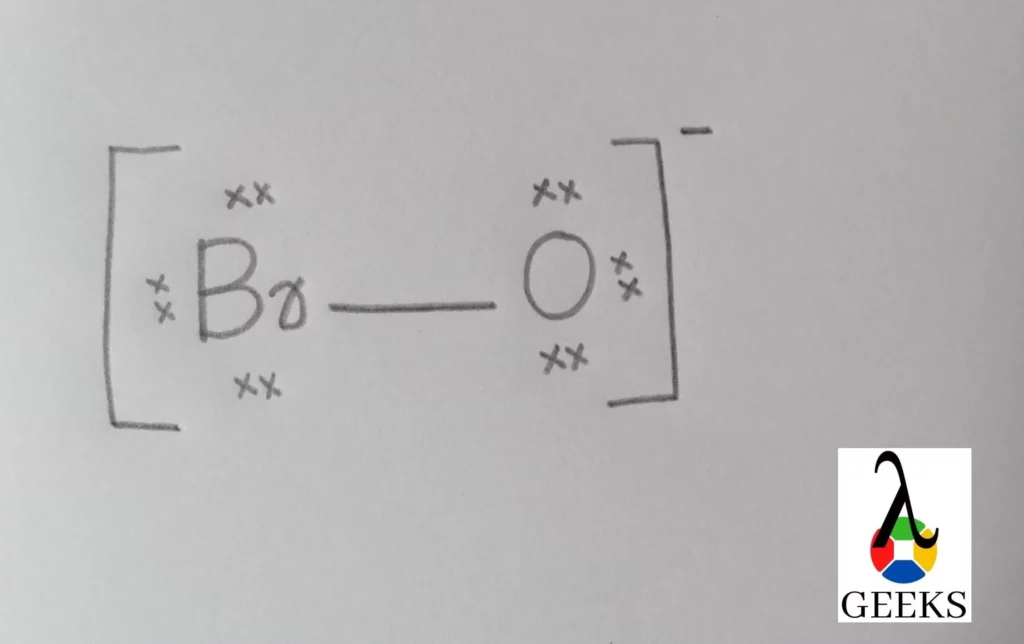PF3Cl2 Lewis Structure & Characteristics (13 Important Facts)
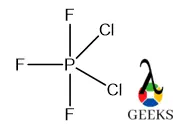
PF3Cl2 or phosphorus dichloride trifluoride is a halogenated compound of phosphorus having a molecular weight of 158.87 g/mol. Let us learn about PF3Cl2. PF3Cl2 is a pentavalent molecule of P, the central P is sp3d hybridized here so the geometry is pentagonal pyramidal. The F atoms are present in that part where s character is … Read more