Xe Lewis Structure & Characteristics: 15 Complete Facts
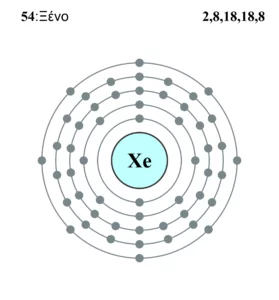
Xe or xenon is a noble gas having molecular weight 131.293 u which belongs to the group 18 of periodic table. Let us discuss the lewis structure and some relevant topics on xenon. Xe possesses a spherical shape due to having uniform electron density around it. It is one of the most soluble noble gases … Read more