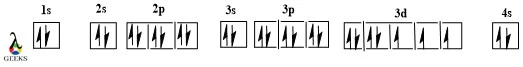
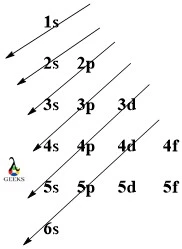
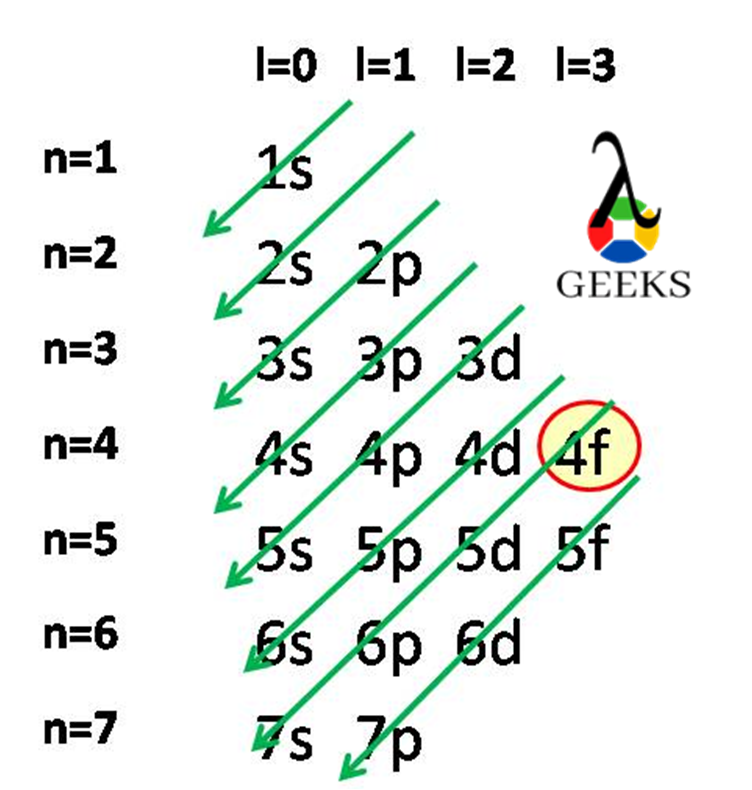
Americium Electron Configuration :7 Easy Steps to Follow!
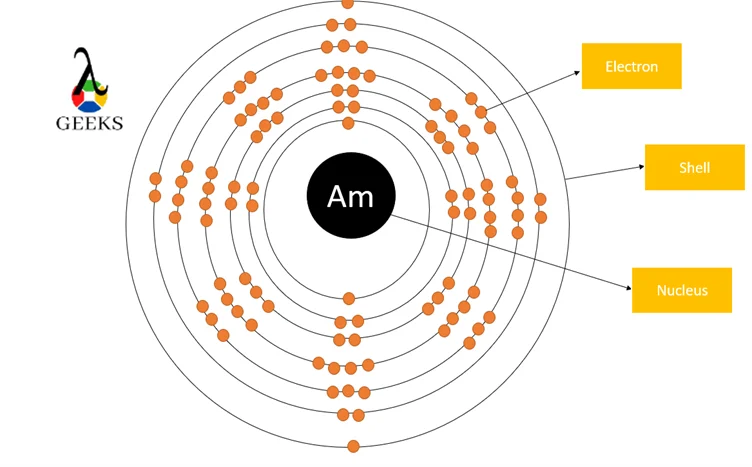
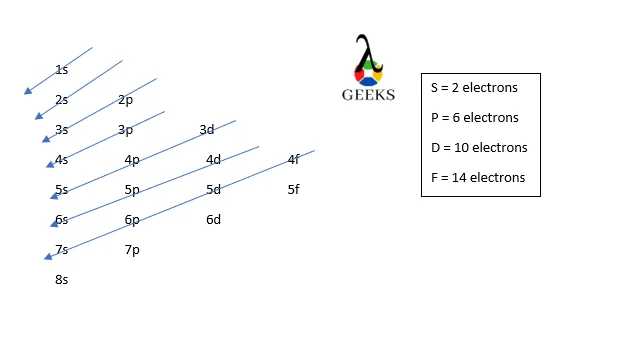
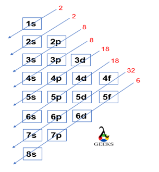
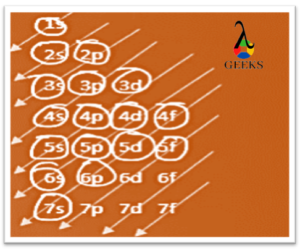
Americium is an actinide series element with the symbol Am. Let us examine some additional details regarding this component. Am electronic configuration: Rn 86 7s2 5f7. Am is a chemical element with the atomic number 95 that is radioactive. It has a metallic luster that is silvery white, but air causes it to tarnish over … Read more