Sulfur trioxide (SO3) features a sulfur (S) atom with 6 valence electrons, forming double bonds with three oxygen (O) atoms, each contributing 6 valence electrons. The Lewis structure shows three S=O double bonds, with 24 bonding electrons and no lone pairs on sulfur. SO3 adopts a trigonal planar geometry with bond angles of 120°, indicative of sp² hybridization. The molecule is nonpolar due to its symmetrical structure, despite the high electronegativity of oxygen (3.44). This arrangement is key to its role in forming sulfuric acid and its high reactivity, particularly in acid rain formation.

Understanding SO3 Lewis Structure
The SO3 Lewis structure refers to the arrangement of atoms and electrons in a molecule of sulfur trioxide (SO3). It provides a visual representation of the chemical bonding and electron distribution within the molecule. By understanding the SO3 Lewis structure, we can gain insights into its molecular geometry, valence electrons, and other important characteristics.
What is SO3 Lewis Structure?
The Lewis dot structure of SO3 involves the sulfur atom (S) bonded to three oxygen atoms (O). Each oxygen atom is connected to the sulfur atom by a double bond, resulting in a total of three double bonds. This arrangement allows for the fulfillment of the octet rule, where each atom has a full outer shell of electrons.
How to Construct SO3 Lewis Structure
To construct the SO3 Lewis structure, we can follow a step-by-step process:
- Determine the total number of valence electrons in the SO3 molecule. In this case, sulfur (S) has 6 valence electrons, and each oxygen (O) atom has 6 valence electrons, giving us a total of 24 valence electrons.
- Identify the central atom, which is sulfur (S) in this case. The oxygen (O) atoms will surround the sulfur atom.
- Place one pair of electrons between the sulfur atom and each oxygen atom to form a double bond. This will account for 12 valence electrons.
- Distribute the remaining 12 valence electrons as lone pairs on the oxygen atoms. Each oxygen atom will have 3 lone pairs.
- Check if all atoms have achieved an octet of electrons. In the case of SO3, each atom has 8 electrons, satisfying the octet rule.
SO3 Lewis Structure Formal Charge

The formal charge of an atom in a Lewis structure is a way to determine the distribution of electrons and the stability of the molecule. To calculate the formal charge, we use the formula:
Formal Charge = Valence Electrons – Lone Pairs – 1/2 * Bonded Electrons
In the SO3 Lewis structure, the formal charge of the sulfur atom is 0, while each oxygen atom has a formal charge of -1. This distribution ensures that the overall charge of the molecule is neutral.
SO3 Lewis Structure Following Octet Rule
The SO3 Lewis structure follows the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with 8 electrons in their outer shell. In the case of SO3, each atom has 8 electrons, fulfilling the octet rule.
SO3 Lewis Structure Bond Angle
The bond angle in the SO3 molecule is approximately 120 degrees. This is due to the trigonal planar molecular geometry, where the three oxygen atoms are arranged symmetrically around the central sulfur atom.
SO3 Lewis Structure Molecular Geometry
The molecular geometry of SO3 is trigonal planar. This means that the molecule has a flat, triangular shape with the sulfur atom at the center and the three oxygen atoms evenly spaced around it. The arrangement of atoms in this geometry minimizes electron repulsion and maximizes stability.
SO3 Lewis Structure Valence Electrons
In the SO3 Lewis structure, there are a total of 24 valence electrons. The sulfur atom contributes 6 valence electrons, while each oxygen atom contributes 6 valence electrons. These electrons are involved in the formation of covalent bonds and lone pairs, determining the overall structure and properties of the molecule.
SO3 Lewis Structure Lone Pairs
In the SO3 molecule, each oxygen atom has 3 lone pairs of electrons. These lone pairs are not involved in bonding and are located on the oxygen atoms. They contribute to the overall electron distribution and stability of the molecule.
By understanding the SO3 Lewis structure, we can gain insights into the arrangement of atoms, electron distribution, and molecular properties of sulfur trioxide. The Lewis structure provides a valuable tool for visualizing and analyzing the chemical structure of molecules.
SO3 Lewis Structure Coordinate Bond
The SO3 molecule, also known as sulfur trioxide, is a compound composed of one sulfur atom and three oxygen atoms. In its Lewis dot structure, sulfur is the central atom, surrounded by the three oxygen atoms. The Lewis dot structure represents the valence electrons of the atoms involved in the molecule and helps us understand the chemical bonding and molecular geometry.
To determine the Lewis dot structure of SO3, we need to follow a few steps. First, we need to determine the total number of valence electrons in the molecule. Sulfur is in Group 6A of the periodic table, so it has 6 valence electrons. Oxygen is in Group 6A as well, so each oxygen atom contributes 6 valence electrons. Therefore, the total number of valence electrons in SO3 is 6 (sulfur) + 3 * 6 (oxygen) = 24.
Next, we need to distribute the valence electrons around the atoms in the molecule. We start by placing a single bond between sulfur and each oxygen atom, which uses up 6 electrons (2 electrons for each bond). This leaves us with 18 electrons to distribute.
To satisfy the octet rule, we place the remaining 18 electrons as lone pairs around the oxygen atoms. Each oxygen atom can accommodate 6 electrons, so we place 2 lone pairs (4 electrons) on each oxygen atom. This gives each oxygen atom a total of 8 electrons, satisfying the octet rule.
The Lewis dot structure of SO3 can be represented as follows:
O
|||
O-S-O
|||
O
In this structure, the sulfur atom is in the center, bonded to each oxygen atom by a double bond. The oxygen atoms have two lone pairs each. This arrangement gives the molecule a trigonal planar molecular shape.
It’s important to note that the Lewis dot structure of SO3 is a resonance hybrid. This means that the double bonds can be interchanged between the sulfur atom and the oxygen atoms, resulting in different resonance structures. The actual structure of SO3 is a combination of these resonance structures, known as a resonance hybrid.
SO3 Lewis Structure Hybridization
In the Lewis dot structure of SO3, the central sulfur atom is surrounded by three oxygen atoms. To understand the hybridization of the sulfur atom, we need to consider its electron configuration and the number of electron pairs around it.
The electron configuration of sulfur is 1s2 2s2 2p6 3s2 3p4. In the formation of SO3, one of the 3s electrons and two of the 3p electrons of sulfur participate in bonding, leaving behind one unpaired electron in the 3p orbital. This unpaired electron is available for bonding with the oxygen atoms.
To determine the hybridization of the sulfur atom, we can use the valence bond theory. The sulfur atom in SO3 undergoes sp2 hybridization, where one 3s orbital and two 3p orbitals combine to form three sp2 hybrid orbitals. These hybrid orbitals are then used to form sigma bonds with the oxygen atoms.
The sp2 hybridization of the sulfur atom in SO3 allows for the formation of three sigma bonds, one with each oxygen atom. The remaining unpaired electron in the 3p orbital of sulfur forms a pi bond with one of the oxygen atoms, resulting in the double bond between sulfur and oxygen.
In summary, the Lewis dot structure of SO3 reveals that the central sulfur atom undergoes sp2 hybridization, forming three sigma bonds and one pi bond with the surrounding oxygen atoms. This hybridization allows for the trigonal planar molecular shape of the SO3 molecule.
Special Cases of SO3 Lewis Structure
SO3H Lewis Structure

The SO3H Lewis structure refers to the chemical structure of sulfur trioxide with an additional hydrogen atom attached. To determine the Lewis dot structure of SO3H, we need to consider the valence electrons of each atom and their arrangement.
In the case of SO3H, sulfur (S) is the central atom, surrounded by three oxygen (O) atoms and one hydrogen (H) atom. Sulfur has six valence electrons, while oxygen has six and hydrogen has one. This gives us a total of 24 valence electrons for SO3H.
To draw the Lewis structure, we start by placing the atoms in a trigonal planar arrangement around the sulfur atom. We then distribute the valence electrons around the atoms, ensuring that each atom has an octet (except for hydrogen, which only needs two electrons).
Next, we check if the octet rule is satisfied for all atoms. In the case of SO3H, the sulfur atom has an incomplete octet. To address this, we can form a double bond between sulfur and one of the oxygen atoms. This allows sulfur to have a total of eight valence electrons, satisfying the octet rule.
The final Lewis structure of SO3H shows a double bond between sulfur and one oxygen atom, and single bonds between sulfur and the other two oxygen atoms, as well as the hydrogen atom.
SO32- Lewis Structure

The SO32- Lewis structure represents the sulfur trioxide molecule with an additional negative charge. This means that there is an extra electron in the structure, which affects the arrangement of the valence electrons.
To determine the Lewis dot structure of SO32-, we start by considering the valence electrons of each atom. Sulfur has six valence electrons, while each oxygen atom has six. Additionally, the negative charge adds one extra electron to the structure, bringing the total to 26 valence electrons.
Similar to the SO3H structure, we arrange the atoms in a trigonal planar shape around the sulfur atom. We then distribute the valence electrons, ensuring that each atom has an octet (except for hydrogen, which only needs two electrons).
In the case of SO32-, the sulfur atom has an incomplete octet. To satisfy the octet rule, we can form a double bond between sulfur and one of the oxygen atoms, and a single bond with the other two oxygen atoms. This allows sulfur to have a total of eight valence electrons.
The final Lewis structure of SO32- shows a double bond between sulfur and one oxygen atom, and single bonds between sulfur and the other two oxygen atoms. The negative charge is represented by an additional lone pair of electrons on the sulfur atom.
SO31- Lewis Structure

The SO31- Lewis structure represents the sulfur trioxide molecule with an additional positive charge. This means that one electron is removed from the structure, affecting the arrangement of valence electrons.
To determine the Lewis dot structure of SO31-, we consider the valence electrons of each atom. Sulfur has six valence electrons, while each oxygen atom has six. Additionally, the positive charge removes one electron from the structure, resulting in a total of 22 valence electrons.
Similar to the previous structures, we arrange the atoms in a trigonal planar shape around the sulfur atom. We then distribute the valence electrons, ensuring that each atom has an octet (except for hydrogen, which only needs two electrons).
In the case of SO31-, the sulfur atom has an incomplete octet. To satisfy the octet rule, we can form a double bond between sulfur and one of the oxygen atoms, and a single bond with the other two oxygen atoms. This allows sulfur to have a total of eight valence electrons.
The final Lewis structure of SO31- shows a double bond between sulfur and one oxygen atom, and single bonds between sulfur and the other two oxygen atoms. The positive charge is represented by the absence of one valence electron on the sulfur atom.
In summary, the special cases of SO3 Lewis structure, including SO3H, SO32-, and SO31-, involve variations in the arrangement of valence electrons due to the presence of additional atoms or charges. These structures can be determined by considering the valence electrons, molecular geometry, and the octet rule.
Properties of SO3 Based on Lewis Structure
SO3 Lewis Structure Polar or Nonpolar

When examining the Lewis structure of SO3, we can determine whether it is polar or nonpolar. The Lewis dot structure of SO3 shows that sulfur (S) is the central atom, bonded to three oxygen (O) atoms. Each oxygen atom is connected to sulfur through a double bond. The Lewis structure also reveals that sulfur has a total of 12 valence electrons, while each oxygen atom contributes 6 valence electrons. By following the octet rule, we can distribute the electrons and determine the molecular geometry.
The molecular geometry of SO3 is trigonal planar, with the sulfur atom at the center and the three oxygen atoms surrounding it. This arrangement results in a symmetrical distribution of electron pairs, making the molecule nonpolar. Despite the presence of polar bonds between sulfur and oxygen, the overall molecular shape cancels out the polarity, resulting in a nonpolar molecule.
Is SO3 Ionic Or Covalent?
SO3 is a covalent compound. Covalent bonding occurs when atoms share electrons to achieve a stable electron configuration. In the case of SO3, sulfur and oxygen atoms share electrons to form covalent bonds. The Lewis dot structure of SO3 clearly shows the sharing of electrons between sulfur and oxygen, indicating a covalent bond.
Is SO3 Reactive with Water?
SO3 is highly reactive with water. When SO3 reacts with water, it forms sulfuric acid (H2SO4). This reaction is exothermic and releases a large amount of heat. The reaction between SO3 and water is as follows:
SO3 + H2O → H2SO4
The reaction between SO3 and water is highly exothermic and can be dangerous if not handled properly. It is important to exercise caution when working with SO3 and water.
Is SO3 Acid or Base?
SO3 is an acidic compound. When SO3 reacts with water, it forms sulfuric acid (H2SO4). Sulfuric acid is a strong acid that dissociates completely in water, releasing hydrogen ions (H+) and sulfate ions (SO4^2-). The presence of hydrogen ions in the solution makes SO3 an acid.
Is SO3 a Lewis Acid or Base?
SO3 can act as both a Lewis acid and a Lewis base. As a Lewis acid, SO3 can accept an electron pair from a Lewis base. This electron pair acceptance allows SO3 to form coordinate covalent bonds. On the other hand, SO3 can also act as a Lewis base by donating an electron pair to a Lewis acid. The ability of SO3 to act as both a Lewis acid and a Lewis base makes it a versatile compound in chemical reactions.
In summary, the Lewis structure of SO3 reveals important properties about the molecule. It helps determine the polarity of SO3, whether it is ionic or covalent, its reactivity with water, and its acidic nature. Additionally, SO3 can act as both a Lewis acid and a Lewis base, showcasing its versatility in chemical reactions.
Advanced Concepts in SO3 Lewis Structure
SO3 Lewis Structure Resonance
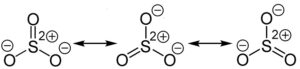
In the study of chemical bonding, the Lewis dot structure is a valuable tool for representing the arrangement of electrons in a molecule. When it comes to Sulfur trioxide (SO3), understanding its Lewis structure becomes particularly interesting due to the presence of resonance structures. Resonance occurs when multiple Lewis structures can be drawn for a molecule, differing only in the placement of electrons. In the case of SO3, resonance allows for the distribution of electron pairs between the sulfur and oxygen atoms, resulting in a more stable overall structure.
To illustrate the resonance in the SO3 Lewis structure, we can examine the electron configuration of sulfur and oxygen. Sulfur has six valence electrons, while each oxygen atom has six valence electrons as well. Following the octet rule, we can distribute these valence electrons around the atoms, starting with a single bond between sulfur and each oxygen atom. This leaves two lone pairs of electrons on each oxygen atom. However, this arrangement does not fully satisfy the octet rule for sulfur.
To achieve a more stable structure, we can utilize resonance to distribute the electron pairs. By moving one of the lone pairs from an oxygen atom to form a double bond with sulfur, we create a resonance structure. This process can be repeated, resulting in multiple resonance structures for SO3. The actual structure of SO3 is a resonance hybrid, which is a combination of all the resonance structures.
Why Does SO3 Have a Double Bond?
The presence of a double bond in the SO3 molecule can be explained by the electron configuration and the concept of formal charges. In the Lewis structure of SO3, each oxygen atom is bonded to the sulfur atom through a single bond. This leaves two lone pairs of electrons on each oxygen atom. However, the octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons.
To satisfy the octet rule for sulfur, one of the lone pairs from an oxygen atom can be moved to form a double bond with sulfur. This results in a more stable structure for SO3, as it allows sulfur to have a complete octet of electrons. The presence of the double bond also contributes to the resonance in the molecule, as discussed earlier.
Why Does SO3 Have 3 Double Bonds?
Contrary to the misconception that SO3 has three double bonds, the actual structure of SO3 involves one double bond and two single bonds. This misconception arises due to the resonance structures of SO3. As mentioned earlier, resonance allows for the distribution of electron pairs between atoms, resulting in multiple possible structures.
In the case of SO3, the resonance structures involve one double bond between sulfur and an oxygen atom, while the other two oxygen atoms are connected to sulfur through single bonds. The resonance hybrid, which represents the actual structure of SO3, is a combination of these resonance structures. Therefore, it is more accurate to say that SO3 has one double bond and two single bonds.
SO3 Lewis Structure VSEPR Theory
The VSEPR (Valence Shell Electron Pair Repulsion) theory provides a framework for predicting the molecular geometry of a molecule based on the arrangement of electron pairs around the central atom. In the case of SO3, the central atom is sulfur, and it is surrounded by three regions of electron density due to the presence of one double bond and two single bonds.
According to the VSEPR theory, the molecular shape of SO3 is trigonal planar. This means that the three regions of electron density around sulfur are arranged in a flat, triangular shape. The bond angles in SO3 are approximately 120 degrees, as the electron pairs repel each other and try to maximize their distance.
In summary, the advanced concepts in the SO3 Lewis structure involve understanding the resonance structures, the presence of a double bond, the misconception of three double bonds, and the molecular shape determined by the VSEPR theory. These concepts provide insights into the chemical bonding and molecular structure of SO3, contributing to our understanding of this important compound.
What are the Uses of Metalloids on the Periodic Table?
The metalloids properties and periodic table play a vital role in various applications. These elements exhibit characteristics of both metals and nonmetals, making them valuable in electronics. Silicon, a metalloid, is widely utilized in semiconductor chips. Boron, another metalloid, finds application in heat-resistant ceramics and alloys. Metalloids like arsenic and antimony are utilized in the manufacturing of glass and pharmaceuticals. These unique properties make metalloids crucial for technological advancements.
Frequently Asked Questions
1. What is the Lewis structure of SO3?
The Lewis structure of SO3, or sulfur trioxide, involves a sulfur atom at the center, surrounded by three oxygen atoms. Each oxygen atom forms a double bond with the sulfur atom, resulting in a total of 24 valence electrons, which satisfies the octet rule.
2. Why does SO3 have a double bond?
SO3 has double bonds because the sulfur atom shares two pairs of electrons with each oxygen atom. This arrangement allows the molecule to satisfy the octet rule, which states that atoms seek to have eight electrons in their outermost electron shell to achieve stability.
3. Is the SO3 Lewis structure polar or nonpolar?
The SO3 molecule is nonpolar. Even though the S-O bonds are polar, the molecule’s trigonal planar geometry ensures that the bond dipoles cancel each other out, resulting in a nonpolar molecule.
4. How to do the SO3 Lewis structure?
To draw the Lewis structure of SO3, start with a sulfur atom in the center and three oxygen atoms surrounding it. Draw a double bond between the sulfur and each oxygen atom. This accounts for 24 electrons – 6 from sulfur and 18 from the three oxygen atoms (6 each), satisfying the octet rule.
5. What is the structure of SO3?
The structure of SO3 is trigonal planar. It consists of a central sulfur atom surrounded by three oxygen atoms, each sharing a double bond with the sulfur. The molecule is symmetrical, with bond angles of 120 degrees.
6. What is the SO3 Lewis structure bond angle?
The bond angle in the SO3 Lewis structure is 120 degrees. This is due to its trigonal planar molecular geometry, which evenly distributes the three oxygen atoms around the central sulfur atom.
7. Why does SO3 have 3 double bonds?
SO3 has three double bonds to satisfy the octet rule, which states that atoms seek to have eight electrons in their outermost electron shell. Each double bond represents two pairs of shared electrons between the sulfur and oxygen atoms.
8. What is the SO3 Lewis structure molecular geometry?
The molecular geometry of the SO3 Lewis structure is trigonal planar. This means the molecule is flat with 120-degree angles between the sulfur-oxygen bonds.
9. Is SO3 a Lewis acid or base?
SO3 is considered a Lewis acid because it can accept electron pairs. This is due to the sulfur atom’s ability to expand its valence shell beyond the octet, allowing it to accept additional electron pairs from other atoms or molecules.
10. What is the SO3 Lewis structure resonance?
The SO3 Lewis structure exhibits resonance, meaning that the double bonds between the sulfur and oxygen atoms can be drawn in multiple ways while still accurately representing the molecule’s structure. This is because the actual structure is a resonance hybrid of the possible structures, with the double bonds distributed equally among the three S-O bonds.
Also Read:
- Cl2co lewis structure
- Co2 lewis structure
- Scl6 lewis structure
- N2 lewis structure
- Zn2 lewis structure
- Sbr6 lewis structure
- Brcl5 lewis structure
- Co32 lewis structure
- Hcch lewis structure
- Sbf5 lewis structure

Hello,
I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202