In this article, we should discuss the SO2Cl2 lewis structure, shape of the molecule, bond angle, and many detailed facts.
SO2Cl2 is known as sulfuryl chloride. The molecule is tetrahedral in shape and has a different bond angle in it. Two Oxygen atoms are attached to central S by a double bond and two Cl are attached via a single bond only. Due to the different environments of bond order, the bond angle is different in this molecule.
The S-O bond length is shorter than S-Cl because of oxygen attached via a double bond and we know a double bond is stronger but shorter than a single bond. The oxidation state of central S in this molecule is +6.
Some important facts about SO2Cl2
SO2Cl2 is a colorless liquid in its physical state at room temperature and the odor of the molecule is pungent. In nature, it cannot exist in its state because the molecule shows rapid hydrolysis. The molar mass of the molecule is 136.96 g/mol. The boiling point and melting point of sulfuryl chloride are 342.5 K and 219.1 K respectively. As it is liquid in its physical state, its refractive index is 1.4437.
Sulfuryl Chloride is synthesized in the laboratory by the reaction of sulfur dioxide and Chlorine in presence of activated charcoal as a catalyst.
SO2 + Cl2 = SO2Cl2
How to draw the lewis structure for SO2Cl2?
With the help of lewis structure, we can predict the molecular shape, the number of electrons involved in bond formation, and the number of lone pairs available of SO2Cl2.

Before drawing the SO2Cl2 lewis structure we should keep in mind some important points. At first, we count the total number of valence electrons of S, O, and Cl atoms. Then we have to identify the central atom on the basis of electronegativity. Among S, O, and Cl less electronegative atom is S, so S is the central atom is here.
In the SO2Cl2 lewis structure, the total number of electrons involved in the bond formation is, 6+(2*6)+(2*7)=32 and the electrons required according to the lewis dot formula is (5*8)= 40 electrons, so the bonding electrons will be (40-32)= 8 electrons and the minimum number of bonds required (8/2)= 4 bonds.
Now we should assign the lone pairs, S has six electrons in its valence shell and 4 electrons are involved in the four sigma bonds and two electrons are involved in two π bonds with two O. So, no lone pairs are available on the S.
SO2Cl2 lewis structure shape
The valence electrons for S involved in sigma bond formation is 4 and the surrounding atoms are two O and two Cl contribute 1 electron each is 4 electrons. So, the total electrons involved in the sigma bond formation is 4+(1*4)=8 electrons.
According to VSEPR (Valence Shell Electrons Repulsion) theory if the electrons count in bond formation for any molecule is 8 then it should adopt tetrahedral geometry.

In the SO2Cl2 lewis structure, the whole electron density lies around the central S atom, and two Cl and two O are present at four sites of the tetrahedral moiety. The ideal bond angle should be 109.50 for tetrahedral but here the scenario is different.
Here both O form a double bond with S so it required more space and due to the larger size of Cl, there is massive lone pair-bond pair repulsion occurs. To minimize this kind of repulsion the molecule adopts different bond angles to arrange every atom.
SO2Cl2 lewis structure formal charges
From the SO2Cl2 lewis structure, we calculate the formal charge assuming the same electronegativity for S, O, and Cl.
The formula we can use to calculate the formal charge, F.C. = Nv – Nl.p. -1/2 Nb.p.
We calculate the formal charge separately for S, O, and Cl because they are different molecules and experience different environments.
The Formal charge over S is, 6-0-(12/2) = 0
The Formal charge over O is, 6-4-(4/2) = 0
The formal charge over Cl is, 7-6-(2/2) = 0
So, we can see that there is no formal charge over any atom in the SO2Cl2 lewis structure. It is also proved that the molecule is neutral in nature so no formal charge over it.
SO2Cl2 lewis structure lone pairs
In the SO2Cl2 lewis structure, only O and Cl contain lone pair over it. S does not have any lone pair because all the valence electrons of S are involved in sigma as well as π bonds.

S is a group VIA element, so it has six electrons in its valence shell. Among six electrons four electrons are involved in the sigma bond formation with two Cl and two o atoms. The rest of the two electrons are also involved in the π bond formation with two O atoms. So, no free electrons are present in the valence shell of S, so S does not have any lone pair.
O is also a group VIA element and it has also six electrons in its valence shell one electron is involved in sigma bond formation with S and another one is an π bond with S. So, the remaining 4 electrons are present as two pairs of lone pairs over O atom.
Cl is a group VIIA element and it has one more electron in the valence shell than O or S which means seven electrons in its outermost orbital. Only one electron is involved in bond formation with S and the rest of the six electrons exist as 3 pairs of lone pairs.
So, the total number of lone pairs available in the SO2Cl2 is (2*2)+(3*2)=10 pairs of lone pairs.
SO2Cl2 Octet rule
According to the octet rule S, O and Cl all are try to complete their valence shell by either donating or accepting the suitable number of electrons in SO2Cl2 lewis structure.

In the SO2Cl2 lewis, structure S make a total of six bond, four sigma bonds, and two π bonds with O and Cl. So, S invests its six electrons to form six bonds and it has nothing in its valence shell. S is a group VIA element and it has six electrons in its valence shell when it forms a bond with two O and two Cl by sharing four electrons then it can complete its octet.
Now for Cl, it has seven electrons in its valence shell and it shares one electron with S to complete its octet.
For O, it has six electrons in its valence shell like S and it forms two bonds with S one is sigma and the other is a π bond by sharing two electrons with S, and this way it also completed its octet and gains the nearest noble gas configuration in SO2Cl2 lewis structure.
SO2Cl2 lewis structure resonance
In the SO2Cl2 lewis structure, the electrons clouds delocalized only between O and S atoms in the different canonical forms via resonance.
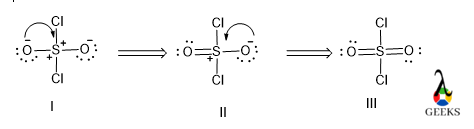
In the SO2Cl2 lewis structure, all of the above are different resonating structures. Among them, Structure III is the most contributing because it has a higher number of covalent bonds. After that Structure II and then least contributing is Structure I as it contains a lower number of covalent bonds and the positive charge is present in the S atom.
SO2Cl2 hybridization
In the SO2Cl2 lewis structure S, O, and Cl undergo hybridization via mixing their orbitals of different energy to form a new equivalent hybrid orbital to form a stable covalent molecule.
We calculate the CCl4 hybridization by using the following formula,
H = 0.5(V+M-C+A), where H= hybridization value, V is the number of valence electrons in the central atom, M = monovalent atoms surrounded, C=no. of cation, A=no. of the anion.
For central atom S in SO2Cl2 lewis structure, S has six valence electrons but four electrons are involved in the sigma bond and surrounding atoms are two Cl and two O atoms.
So, the hybridization of central S is, ½(4+4+0+0) = 4(sp3)
| Structure | Hybridization value | state of hybridization of central atom | Bond angle |
| Linear | 2 | sp /sd / pd | 1800 |
| Planner trigonal | 3 | sp2 | 1200 |
| Tetrahedral | 4 | sd3/ sp3 | 109.50 |
| Trigonal bipyramidal | 5 | sp3d/dsp3 | 900 (axial), 1200(equatorial) |
| Octahedral | 6 | sp3d2/ d2sp3 | 900 |
| Pentagonal bipyramidal | 7 | sp3d3/d3sp3 | 900,720 |
From the above table of hybridization, we can conclude that if the hybridization value is 4 then the central atoms is sp3 hybridized.
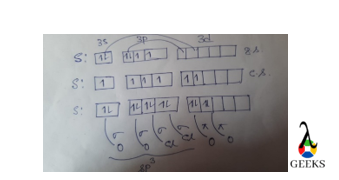
Form the box diagram we can say that we only consider the sigma bond in hybridization not the π bond.
Here One s and three p orbitals of S are involved in the sp3 hybridization.
Is SO2Cl2 polar or nonpolar?
In the SO2Cl2 lewis structure, all the molecule is not opposite to each other so it has a resultant dipole moment and the molecule is polar.

In the SO2Cl2 lewis structure, the dipole moment acts from S to O and S to Cl atoms as Cl and O both are more electronegative than S.
In the tetrahedral moiety, the shape is not symmetric so each molecule’s position is not opposite to each other and each has a specific dipole moment as a result the molecule has a specific value of dipole moment and makes the molecule polar.
From the above discussion of SO2Cl2 lewis structure, we can say that the molecule is polar having a resultant dipole moment. The double bond requires more space than a single bond otherwise the molecule suffers massive bond pair lone pair repulsion.
Also Read:
- Nabr lewis structure
- Cl2o2 lewis structure
- Li lewis structure
- Ibr3 lewis structure
- Brcl3 lewis structure
- Ch3cl lewis structure
- K2co3 lewis structure
- Fecl2 lewis structure
- Cbr4 lewis structure
- Asf5 lewis structure

Hi……I am Biswarup Chandra Dey, I have completed my Master’s in Chemistry from the Central University of Punjab. My area of specialization is Inorganic Chemistry. Chemistry is not all about reading line by line and memorizing, it is a concept to understand in an easy way and here I am sharing with you the concept about chemistry which I learn because knowledge is worth to share it.