Lanthanum is represented by the symbol “La.” Let us predict the electronic configuration of Lanthanum in this article.
The electronic configuration of “La” is 1s22s22p63s23p63d104s24p64d105s25p65d16s2. Lanthanum is an f-block element. It belongs to period 6 of the periodic table. It has an atomic weight of 57. It shows a double hexagonal close-packed crystal structure.
We will focus on various facts about lanthanum electronic configuration, lanthanum electronic configuration diagram, and more such facts in this article.
How to write the lanthanum electron configuration
Electron configuration can be written step by step as given below.
- The first step is finding the shell number.
- Lanthanum is found with 6 electron shells.
- In the second step, orbitals are found.
- S, p, d and f are the four orbitals that hold electrons.
- Orbital with maximum electron holding capacity is given below.
| Orbital | Maximum electron holding capacity |
|---|---|
| s | 2 |
| p | 6 |
| d | 10 |
| f | 14 |
- This is an important step where the orbital is filled with electrons according to the Aufbau principle in increasing the order of energy level of the orbital.
- The filling of electrons in orbital starts with the least energy orbital which is 1s orbital.
- Then following Hund’s rule electrons get paired with their spin facing opposite directions followed by Pauli Exclusion Principle.
- An electron in an orbital can be represented in the form of a superscript. Example 2p6 here superscript 6 reflects a number of electrons.
Thus, the Lanthanum configuration is :
1s22s22p63s23p63d104s24p64d105s25p65d16s2
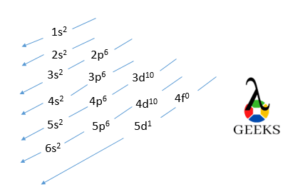
Lanthanum electron configuration diagram
The electronic configuration of Lanthanum is drawn in the form of the diagram as mentioned below.
- 1s orbital which has a maximum capacity of two electrons gets filled first.
- Then 2s orbital is filled with the maximum capacity of two electrons.
- After 2s electron enter 2p orbital with a maximum of six electrons
- After 2p, 3s, 3p, and 4s orbital with a capacity of two, six, and two electrons are filled.
- Then electrons enter in 3d orbital which has a maximum capacity of holding 10 electrons.
- After this, 4p, 5s, 4d, 5p, 6s, and 5d orbital filled in the same way as above.
Lanthanum electron configuration notation
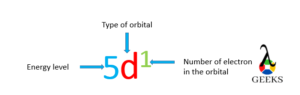
Lanthanum electron configuration notation is : [Xe] 6s2 5d1 .
Lanthanum unabbreviated electron configuration
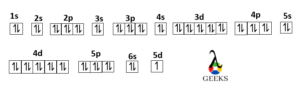
Lanthanum unabbreviated electron configuration is : 1s2 2s2 2p6 3s2 3p6 4s2 5d10 4p6 5s2 4d10 5p6 6s2 5d1
Ground state lanthanum electron configuration
The ground state has low energy so, it is a stable state. Ground state electronic configuration of Lanthanum is :
The excited state of lanthanum electron configuration
The excited state of Lanthanum is predicted as [Xe] 6s1 5d2. The excited state is a higher energy state. A laser resonance ionization time-of-flight spectrometer is used to find out the highly excited state of Lanthanum. Here one electron from 6s paired orbital shifts to the 5d orbital.
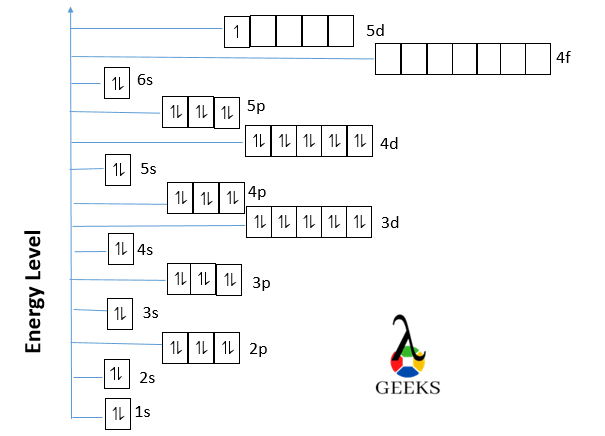
Ground state lanthanum orbital diagram
The ground state orbital diagram of Lanthanum is drawn below :
Conclusion
The electronic configuration studies how electrons are distributed in different orbitals depending on their energy level. Lanthanum contains a total of 57 electrons in its orbital. Lanthanum is used in kidney disease. It is used in making alloys. It is used in making carbon arc light.
Also Read:
- Thulium electron configuration
- Hassium electron configuration
- Dubnium electron configuration
- Scandium electron configuration
- Actinium electron configuration
- Uranium electron configuration
- Germanium electron configuration
- Argon electron configuration
- Phosphorus electron configuration
- Yttrium electron configuration

Hello everyone I am Arti D. Gokhale. My master’s with a specialization in Organic Chemistry and I graduated with Chemistry, Biology, and Zoology. I have 3 years of work experience in water analysis at the National Environmental Engineering Research Institute Nagpur.