In this article we want to discuss about the if2- lewis structure including its drawing, hybridization, shape, charges, pairs and some FAQS.
IF2– is a poly halide in which iodine act as a central atom and 2 fluorine atoms act as terminal atom.
IF2– Lewis Structure Drawing
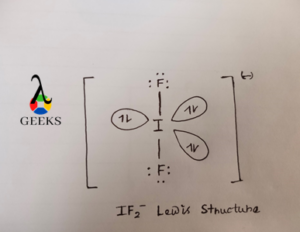
In the if2- lewis structure we see that as iodine becomes larger in size and less electronegative than F it goes in the center of the Lewis structure.
Iodine has 7 valance electrons out of which 2 electrons take part in bonding with F atom and form 2 covalent sigma bond and still 3 electron pair there on I atom which does not take part in bonding F atom and exist as lone pair of electron. F has also 7 electron in their valance shell out of which only 1 electron make covalent bond with central I atom and remaining 6 electrons present as lone pair on I atom.
IF2– Lewis Structure Resonance
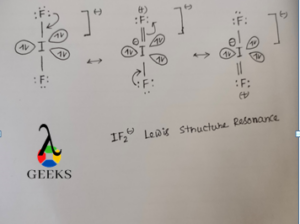
Resonance means shifting of electron pair from one atom to another atom and the structure obtained by this process is called resonating structure.
IF2– has 3 resonating structure in which each I-F bond gets partial double bond character by the process in which F atom donate its lone pair of electron into vacant d orbital of I atom to form p(pi)-d(pi) back bonding.
IF2– Lewis Structure Shape
According to VSEPR theory the shape of IF2–is linear in which iodine is the central atom around which 2 F atoms surround it. As 3 lone pair present in the central I atom the ideal TBP geometry becomes distorted. If 3 lone pair is not present in IF2– the shape becomes TBP but as 3 lone pair present on central I atom the shape becomes distorted and the actual shape is linear.
IF2– Lewis Structure Formal Charge
The formal charge on any atom can be calculated by the formula given below:
Formal Charge (f) =V-B/2-N
Where,
V= No of valance electrons, B= No of bonding electrons, N= No of nonbonding electrons.
Hence formal charge on I atom in IF2–=7-4/2-6= -1
Formal charge on each F atom in IF2– =7 -2/2-6= 0
Hence formal charge on I atom is -1 and on each F atom is 0 making the whole compound is negatively charged.
IF2– Lewis Structure Angle
if2- lewis structure has distorted TBP geometry hence normal TBP bond angle 1200 and 900 does not arises here. Here in this compound lone pair will occupy the equatorial position and F atoms will occupy the axial position.
This is because according to Bent’s rule more electronegative atom will occupy the hybrid orbital having less s character and less electronegative atom will occupy the hybrid orbital having more s character.
Hence we know that lone pair has 0 electronegativity for that it will occupy equatorial position where % of s character is 33.3 and more electronegative F atom will occupy the axial position where % of s character is 0 making the F-I-F bond angle is 1800.
IF2– Lewis Structure Octet Rule
In if2- lewis structure we see that each F atom has 8 valance electrons and completes their octet. In I forms 2 I-F bonds and each bond contain 2 electrons. There is also 3 lone pairs that present on I atom making a total of 10 electrons around I atom. As I is a member of 3rd period and we also know that 3rd period element can increase their octet more than 8 electrons. Hence according to octet rule IF2– is a stable compound.
IF2– Lewis Structure Lone Pairs
The valance electron that does not take place in bonding is defined as lone pair of electron or nonbonding electrons.
The formula through which we can calculate the lone pair of electron is given below:
For central atom,
No of lone pairs=Total no of valance electron of the atom-no of bonding electron formed by that atom
In if2- lewis structure , lone pair present on I atom=7-4=3
For terminal atom,
No of lone pairs=Total no of valance electron-no of bonds formed by that atom
Lone pair present on each F atom=7-1=6 i.e. 3 lone pair
These lone pairs are shown In the Lewis structure of IF2– on the given atoms as dots.
IF2– Valance Electrons
At first, to calculate the total valance electron in IF2–, it is essential to know the electronic configuration of I and F atom.
The electronic of I is [Kr36]4d105s25p5 and from electronic configuration we see that there are 7 electrons in the outermost shell of I atom. The electronic configuration of F atom is [He2]2s22p5 and has 7 electrons. As both I and F both belong to same group i.e. 17, there are 7 valance electrons in both I and F atom. There is also 1 negative charge.
The total valance electron in the compound will be equal to the sum of the valance electron of I and F atom + 1 negative charge i.e. (7*1) + (7*2) + 1=22. There are 22 valance electrons in this species.
IF2– Hybridization

Hybridization is the process of mixing of same energy atomic orbitals to form an equal number of hybrid orbitals.
The ground state valance shell electronic configuration of I is 5s25p5. In the ground state of I we see that there is only 1 unpaired electron and to make IF2– 2 unpaired electron is required. In the excited state I send its 1 p electron into d orbital making a total of 3 unpaired electrons. In the next step 2 F atoms give their 1 unpaired electron to form 2 I-F sigma bonds and sp3d hybridization takes place according to VSEPR theory.
In this compound I uses sp3d hybrid orbital to make I-F bonds. According to sp3d hybridization the geometry should be TBP but the actual structure is linear due to the presence of 3 lone pair of electron in the equatorial position of if2- lewis structure.
IF2– Uses
if2- mainly used in making eye drops. It is used as a fluorinating agent. It is used in explosive material.
FAQS about IF2–
Is IF2– Ionic or Covalent?
IF2– is a covalent compound. This is because it is formed by covalent sigma bonds. In the formation of ionic compound there occurs shifting of electron from electropositive atom to electronegative atom. In if2- lewis structure it is not possible because there occurs mutual sharing of electron between I and F atom to form sigma bonds so that no ions is formed. Hence it is a covalent compound.
Is IF2– Stable?
IF2– is an unstable compound. This is due to the presence of 3 lone pair electron around I atom and there occurs severe LP-LP repulsion. Also I-F bonds are not too strong due to large electronegativity difference and poor orbital overlap between I and F atoms. Due to these two reason this compound is not stable.
Is IF2– polar in nature?
IF2– is a nonpolar compound. A compound is said to be polar when its dipole moment is not equal to 0. The electronegativity of I and F is 2.66 and 3.98 respectively that’s why I-F bond moment lies towards F atom. But as the shape is linear, 2 I-F bond moments lie in opposite direction and cancel each other making the molecule nonpolar.
Also Read:
- Hno lewis structure
- No2f lewis structure
- Hf lewis structure
- Krf4 lewis structure
- Sicl2br2 lewis structure
- Kno3 lewis structure
- Gai3 lewis structure
- Cai2 lewis structure
- Bro3 lewis structure
- Caco3 lewis structure

Hi….I am Susanta Maity. I have completed my Masters from Vidyasagar university with a specialization in organic chemistry.
I love to write complicated Chemistry concepts in understandable and simple words.
Let’s connect through LinkedIn: