Electrical energy can be converted into chemical energy indirectly. Most commonly electrolysis is used for the conversion of electrical to chemical energy.
A list of example of electrical energy to chemical energy is given below:
- Rechargeable flashlight
- Charging the mobile phones
- Power banks
- Electrolytic capacitors
- Electroplating
- Electrolysis of water
- Welding
- Current cathodic protection
- Making of memory chip
- Cyclotron
Explanation on the example of electrical energy to chemical energy
The electrochemical reaction is the process in which a chemical reaction takes place in the presence of electricity as a catalyst, in which electrical energy is converted into chemical energy.
Rechargeable flashlights
The rechargeable flashlight consists of a battery to store the charges. When the flashlight is connected to the AC power supply, the electric charges flow towards the batteries to convert electrical energy into chemical energy.
The batteries are made up of lithium and nickel ions, and also an electrolyte is used in the battery. The electrolyte allows the charges to pass between the cathode and anode terminal. Hence, the electric charges from the power supply stored in the battery of the flashlight possess chemical energy.
Charging the mobile phones
Basically, mobile phones are working as a charging and discharging process. The battery of the mobiles is made of lithium polymer consisting of the lithium-ion electrode.
When you charge your phone, the charges from the power supply move towards the battery. The electrons from the charger move towards anode from the cathode of the battery, thus storing the charges possessing chemical potential energy. Hence charging your phone is an excellent example of electrical energy to chemical energy.

Power banks
When you are travelling, one of the essential things you carry is the power bank used to charge your phone. When the power bank is charging, the electric charges are stored inside the battery through the electrolysis process. Thus charges possess the chemical potential energy to reside in the power bank.
Read more about conversion of mechanical energy to chemical energy
Electrolytic capacitors
Capacitors are the electric components used for storing the electrical charges inside the electric field. Electrolytic capacitors are used in the operation of high voltage DC supply.
The electrolytic capacitors are of asymmetric construction; hence they are polarized. It consists of an anode made up of an oxidized insulating layer that acts as a dielectric. The electrolyte used in the capacitor acts as the cathode. The electric charges from the power supply are now stored inside the capacitor through the electrolysis thus electrical energy of the charges possesses chemical energy.
Electroplating
Electroplating is a process of coating the metal surface by negative charge metal by immersing them in the solution of metal salt. One metal is considered an anode, and the other, which needs to be coated, serves as a cathode.
An external source is connected to the metal to reduce the metal cation. As the current is passed, the cathode terminal undergoes a reduction reaction, and the anode undergoes an oxidation reaction. The metal is dissolved in the anode is plated on the cathode surface. Thus electrical energy from the external source is converted into chemical energy.
The gold plating on the metallic jewellery is done by electroplating. Electroplating is used to prevent the corrosion of the metal.
Electrolysis of water
Electrolysis of water is the most commonly explained example of electrical energy to chemical energy. It is a process of separating hydrogen gas from water by applying external current.
Water electrolysis is done by connecting the DC power supply to two inert electrodes such as platinum or indium immersed in the water. As the current passes through the electrodes, hydrogen settles down at the cathode, and oxygen will be at the anode. Thus electrical energy is responsible for the breaking the bond between the water molecule and causing the separation of hydrogen and oxygen molecule.
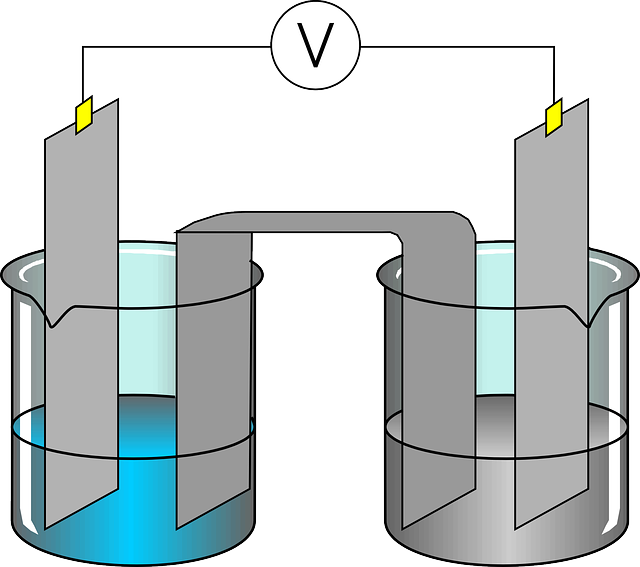
Welding
Welding is the process of melting a part of two metals or thermoplastics to join them together. An external current source is connected to the welding machine, as the current flows the welding gun heats up resulting in the melting of the metal. Allow them to cool for some time, causing fusion. Thus both metals are held by a strong bond possessing chemical energy.
In welding, electrical energy is initially converted into thermal energy, causing chemical energy.
Current cathodic protection
The cathodic protection sounds similar to electroplating, but the application differs from one another.
Cathodic protection is an electrolytic reaction in which more electropositive metal serves as the anode while electronegative as a cathode. Both anode and cathode metal are immersed in water or soil. Then, the current is passed through an external source; the cathode undergoes reduction, and the anode undergoes oxidation; thus, the metal is prevented from corrosion. The chemical reaction takes place by applying electric current; thus, electrical energy is converted into chemical energy.
Read more on conversion of chemical energy to mechanical energy
Making of memory chip
To store the data, we use memory chips are the best example of electrical energy to chemical energy.
The memory cards are made up of silicon wafers. There are several electrical components like transistors are involved in the chip. Suppose we input any data, the transistor switches and turns on. This data pattern accumulates on the silicon wafer, forms the multilayer interconnected lattice pattern, and stores the data. The stored data possess chemical potential energy.

Cyclotron
A cyclotron is an accelerator which accelerates the charged particles. By the application of an electric field to the cyclotron, the acceleration of the particle keeps on increasing, thus initiating the production of radioactive isotopes. Thus the electrical energy is responsible for producing the new isotopes. Hence cyclotrons are a good example of electrical energy to chemical energy.

Read more on Conversion of chemical energy to kinetic energy
Also Read:
- Electrical energy to chemical energy
- Gravitational energy to mechanical energy
- Is potential energy stored energy
- Energy and gravity
- How to calculate energy in ferromagnetic materials
- How to calculate energy required for ionization in plasma
- How to design radiant energy efficient daylighting systems in buildings
- How to determine energy spread in a beam of particles
- Is kinetic energy conserved in an inelastic collision
- Conservation of mechanical energy examples
I am Keerthi K Murthy, I have completed post graduation in Physics, with the specialization in the field of solid state physics. I have always consider physics as a fundamental subject which is connected to our daily life. Being a science student I enjoy exploring new things in physics. As a writer my goal is to reach the readers with the simplified manner through my articles.