In this article, “e1 reaction examples” different type of examples on E1 reaction with detailed explanations are discussed briefly.
The examples are–
- Acid catalyzed Dehydration of Secondary Hydroxyl Group
- Acid Catalyzed Dehydration of Tertiary Hydroxyl Group
- Dehydrohalogenation of Secondary Alkyl Halide
- Dehydrohalogenation of Tertiary Alkyl Halide
- Pyrolysis of a Sulfonate Ester of Methanol
What is an Elimination Reaction?
Elimination reaction is a well known reaction in organic chemistry in which two substituents are removed from one compound followed by either one step or two step mechanism.
E1 reaction (unimolecular elimination reaction) is those type of elimination reaction follows first order kinetics. In rate determining step only one molecular species participate in reaction that is the decomposition step of leaving group from substrate.
E1 reaction proceeds through the following steps-
- Ionization step: carbocation is formed after the elimination of leaving group.
- Deprotonation step: H+ gets eliminated from another carbon beside the leaving group attached carbon.
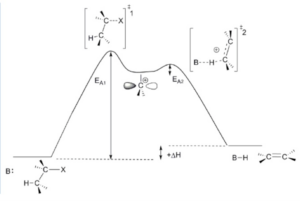
Image Credit: Wikimedia Commons
Acid Catalyzed Dehydration of Secondary Hydroxyl Group
This reaction proceeds through E1 pathway. At the very first step hydroxyl group gets protonated in presence of any concentrated acid and form good leaving group (OH2+). This leaving group can be easily eliminated from the substrate forming a stable carbocation. At the final step deprotonation occurs, one H+ atom is eliminated from the carbon beside the carbon attached with leaving group to form the elimination product.
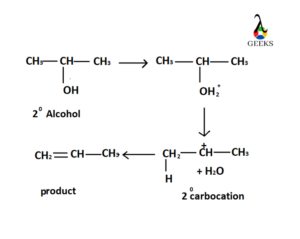
Acid Catalyzed Dehydration of Tertiary Hydroxyl Group
This dehydration process is almost same as the dehydration of secondary hydroxyl group. The difference is that the carbocation generated in this dehydration process is more stable than the previous (30 carbocation is more stable than 20 carbocation). Rest of the mechanism is exactly same.

To know more please go through: 10+ Covalent bond types of elements: Detailed Insights And Facts
Dehydrohalogenation of Secondary Alkyl Halide
Dehydrohalogenation of secondary alkyl halide in presence of base like t-butoxide. At the very first stage halide ion (Cl, Br, I) eliminates and secondary carbocation is generated. In the next step, this reaction base eliminates the hydrogen as H+ from the substrate to form the elimination product (alkene). This secondary carbocation is more stable than primary but less stable than tertiary carbocation.
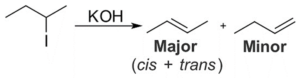
Image Credit: Wikimedia Commons
To know more please check: Alkyl Halide Examples: Detailed Insights And Facts
Dehydrohalogenation of Tertiary Alkyl Halide
This dehydrohalogenation process follows the same mechanism as the previous example. Halogen is eliminated and a tertiary carbocation is generated in the reaction medium in the rate determining step (slowest step). Basic medium helps to remove the hydrogen ion (H+) from the substrate and two types of alkenes is formed Zaitsev (more substituted alkene) and Hofmann alkene (less substituted alkene).

Image Credit: Wikimedia Commons
To know more please follow: SN1 mechanism: Detailed Insights And Facts
Pyrolysis of a Sulfonate Ester of Methanol
Pyrolysis of aryl sulfonate ester takes place through E1 mechanism. In this example carbocation is generated when the sulfonate ester C-O bond is cleaved. This reaction proceeds at very low temperature. This low temperature helps to produce clean and high yielded desired alkene as the product. The aryl sulfonate that is chosen for this pyrolysis should have only one β hydrogen atom in anti -periplanar configuration with the C-O bond. This reaction proceeds in basic medium to eliminate the hydrogen as H+ ion.
Stereoselectivity and Regioselectivity of E1 Reaction
In E1 reaction generally two types of alkenes are formed. One is more substituted alkene known as Hofmann product and another one is less substituted alkene known as Zaitsev’s product. More substituted product has greater stability with respect to less substituted product. This indicates that the hydrogen comes from most substituted carbon will be deprotonated first than the hydrogen belongs to less substituted carbon.
From the mechanism of E1 reaction it is clear that it is not stereospecific like E2 reaction. For E2 reaction deprotonating hydrogen should be antiperiplanar to the leaving group. But, this criteria may not be followed for E1 reaction.
To know more please check: Stereoselective vs Stereospecific: Detailed Insights and Facts
Kinetic Isotope Effect involved in E1 reaction
Kinetic isotope effect is defined as the change of the rate of reaction due to change of one atom by its isotope.
E1 reaction exhibit small primary kinetic isotope effect. When the bond between alpha carbon and hydrogen (Cα-H) is replaced by the heavier isotope of hydrogen (Dueterium) it exhibits secondary kinetic isotope effect and it depends on the stability of carbocation. This isotope effect occurs because the bond strength of C-D bond is almost seven times stronger than C-H bond.
Frequently Asked Questions (FAQ)
Which factor E1 reaction depends on?
Answer: E1 reaction is unimolecular elimination reaction. It depends only on the concentration of substrate because the rate determining step of E1 reaction is the formation of carbocation.
What is an E2 reaction?
Answer: E2 reaction is one type of bimolecular elimination reaction. The rate of an E2 reaction depends on the concentration of substrate as well as the concentration of reagent.
Which substrate is favored for E1 reaction?
Answer: Substrate having tertiary group is favored for E1 reaction because tertiary is most stable carbocation with respect to secondary and primary carbocation due to hyperconjugation effect.
Also Read:
- Anaerobic respiration examples
- Examples of evaporation
- Dipole dipole forces examples
- Non contact force examples
- Refraction of sound examples
- Electrostatic force examples
- Sn2 examples
- Osmosis examples
- Static friction examples
- Neutral equilibrium examples

Hello,
I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202