The main purpose of using a nozzle is to accelerate the velocity of a flowing fluid using pressure. In this article we will discuss about Isentropic Efficiency of Nozzle.
Isentropic efficiency of nozzle is the ratio actual kinetic energy at nozzle exit and isentropic kinetic energy at nozzle exit for the same inlet and exit pressures.
A fluid accelerates in a nozzle as it is moving from high pressure to low pressure with an increase in kinetic energy. Frictional losses inside the nozzle decreases fluid KE and raise the temperature of the fluid, increasing its entropy.
Nozzles are operated under adiabatic condition but the ideal process for a nozzle is the isentropic process. To have a comparison between actual work done and work under isentropic conditions of a device, a parameter called Isentropic Efficiency is used.

What Is Isentropic Efficiency of Nozzle?
The isentropic process involves no irreversibilities and serves as the ideal process for adiabatic devices.
Turbines, compressors and nozzles works under adiabatic conditions. Since they are not truly isentropic, they are considered as isentropic for calculation point of view. Isentropic efficiency is the parameter for a nozzle, turbine or compressor which defines how efficiently these devices approximate a corresponding isentropic device.
Nearer to an idealized isentropic process, improved will be the performance of the nozzle.
IsentropicEfficiency of nozzle is generally greater than 95%. So losses due to irreversibilities are very small in case of a well designed nozzle.
What is a Nozzle?
Nozzles are most widely used steady flow device in steam turbines, gas turbines and rockets.
Nozzle is a device often a pipe or a tube of varying cross sectional area used to control the direction of flow as well as exit velocity, mass, shape and pressure of the flow. Inside a nozzle pressure energy is converted into kinetic energy or we can say the fluid velocity increases with an expense of pressure energy.
Depending on required velocity and mach number of the fluid, Nozzles can be categorised like Convergent type, Divergent type and Convergent-Divergent type. Nozzle can be used for both subsonic and supersonic flows.
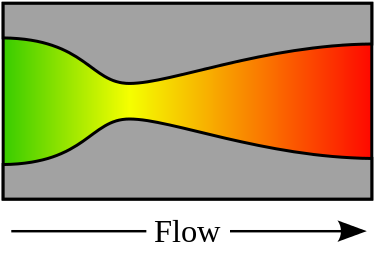
In the above figure, a de Laval nozzle, showing approximate flow velocity increasing from green to red in the direction of flow
Isentropic Efficiency of Nozzle Formula
Isentropic Efficiency represents the performance index of a nozzle. A comparison of nozzle’s performance relative to an isentropic process.
Isentropic Efficiency of Nozzle can be defined as the ratio of actual enthalpy drop to isentropic enthalpy drop between the same pressures.
Isentropic Efficiency of Nozzle=Actual enthalpy drop/Isentropic enthalpy drop
Isentropic Efficiency formula is the measure of the deviation of actual processes from the corresponding idealized ones. The ratio of actual work done by a nozzle to work done by the nozzle under isentropic condition is called Isentropic Nozzle Efficiency.
Isentropic Efficiency of a nozzle ηN= Actual Kinetic Energy at Nozzle Exit/ Isentropic Kinetic Energy at Nozzle Exit.
Theoretically the process inside the nozzle is considered as isentropic but due to frictional losses the process is irreversible.

Process 1-2:Isentropic Process
Process1- 2{}’:Actual Process
Efficiency of nozzle,
For Process 1-2, applying SFEE,
Or,
For Process 1- 2′, applying SFEE,
Or,
Now from Eq(1) substituting the values of h1 – h2 and h1 – h2` ,we get
Equation(1) and (4) are the formulas to calculate the Isentropic Efficiency of Nozzle.
How to Find Isentropic Efficiency of Nozzle?
A Nozzle reduces the pressure of the flow and at the same time speed up the flow to create a thrust.
Some amount of heat loss takes place from the steam due to the friction with the surface of the nozzle. Frictional effect also increases the dryness fraction of steam, because energy lost in friction is transferred into heat which tends to dry or super heat the steam.
In case of fluid dynamics, stagnation point denotes a point where local velocity of a fluid remains zero and isentropic stagnation state represents a state when a flow of fluid goes through reversible adiabatic deceleration to zero velocity.
Both actual and isentropic states are used for gases.

The actual stagnation state is obtained for actual deceleration to zero velocity, irreversibility may be also associated. For this reason stagnation property is sometimes reversed for actual state properties, and the term total property is applied for isentropic stagnation states.
Both isentropic and actual stagnation states have same enthalpy, same temperature(for ideal gas) but may be pressure is more in case of isentropic stagnation state in comparison to actual stagnation state.
In case of a nozzle the inlet velocity is negligible in comparison to exit velocity of a flow.
From the energy balance,
Isentropic Efficiency of Nozzle=Actual enthalpy drop/Isentropic enthalpy drop
Where h1 =specific enthalpy of the gas at the entrance
h2a =specific enthalpy of gas at the exit for the actual process
h2s = specific enthalpy of gas at the exit for the isentropic process
Isentropic Efficiency Nozzle Example
Example: Steam enters a nozzle at 1.4 MPa 2500 C and negligible velocity and expands to 115 KPa and a quality of 97% dry. Determine the exit velocity of the steam.
Solution: Given data , Initial Pressure, P1=1.4MPa
=14 bar
Initial Temperature, T1=2500 C
Final Pressure,P2=115 KPa= 1.15 x 105 Pa=1.15 bar
Quality of steam at exit, x2=0.97
Exit Velocity, V2=?
Neglecting initial velocity, Exit Velocity,
Considering initial velocity,
h1=Enthalpy at initial condition i.e. at 1.14 MPa i.e at 14 bar 2500C, from steam tables,
h1=2927.6 KJ/Kg
h2=Enthalpy at exit condition i.e. at 115 KPa i.e at 1.15 bar x2=0.97, from steam tables
hf2=434.2 KJ/kg
hfg2=2247.4 KJ/kg
Hence the exit velocity of steam,