NOBr is the chemical formula of Nitrosyl Bromide. It is a reddish coloured gas. Let us discuss some important facts about it.
The Lewis structure of NOBr consists of central atom Nitrogen. It is bonded with Bromine with a single bond. There is double bond between Nitrogen and Oxygen. There is one lone pair on N, 2 lone pairs on
Oxygen and 3 lone pairs on Bromine in the Lewis structure of NOBr.
Let us discuss Lewis structure and characteristics of NOBr in brief below.
How to draw NOBr Lewis structure?
Lewis structure compound. Let us discuss the Lewis structure of NOBR below.
Step 1: Determine total valence electrons
The valence electrons in Nitrogen, Oxygen and Bromine are 5, 6 and 7 respectively. Thus, total valence electrons in NOBr = 5 + 6 + 7 = 18 electrons.
Step 2: Select the central atom for NOBr
Nitrogen being the least electronegative atom goes in the center of the Lewis structure of NOBr.
Step 3: keep two electrons to represent a chemical bond
Out of !8, four electrons are used up in forming single bonds between the atoms.
Step 4: Complete the octet of the surrounding atoms atoms and valence electrons which are left put them on central atom
Complete the octet of Oxygen and Bromine. On completing the octet of outer atoms, we found that only 16 valence electrons are used. There are total 18 valence electrons out of which 2 electrons are left. Put the remaining 2 electrons on Nitrogen.
Step 5: Complete the octet of the central atom
There are only 6 electrons on central Nitrogen atom. Now, move the electron pair from outer Oxygen atom to complete the octet of the central atom.
Step 6: Formal charge calculation
Formal charge for each atom in the Lewis structure of NOBr is zero. Thus, we have got the best structure for NOBr.
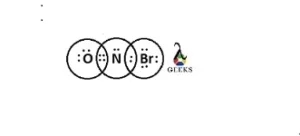
NOBr Lewis structure shape
The shape of molecules can be determined by knowing bonding pairs and lone pairs on the center atom and using the VSEPR theory. Let us explore below.
NOBr has a bent molecular geometry and electron geometry as trigonal planar. The molecular geometry is determined by bonding pairs as well as the lone pairs. The electron geometry is determined by bonding pairs.
NOBr Lewis structure formal charge
Formal charge is a way of keeping track of electron distribution in a Lewis structure of a molecule or a polyatomic ion. Let us discuss the formal charge of NOBr below.
Formal charge over each atom in Lewis structure of NOBr is zero. The formula to calculate formal charge is, Formal charge = number of valence electrons – number of non-bonded electrons – 0.5*number of bonded electrons
Let us calculate the formal charge for each atom in NOBR below.
- For the central atom N atom = 5 – 2 – 0.5*6 = 0
- For the double bonded O atom = 6 – 4 – 0.5*4 = 0
- For the single bonded Br atom = 7 – 6 – 0.5*2 = 0
NOBr Lewis structure angle
Bond angle is decided by the hybridization of the central atom. Let us discuss the bond angle for NOBr below.
The bond angle in NOBr is 120 degrees. The molecular geometry of NOBr is bent and there is lone pair present on the central atom. This makes bond angle 120 degrees.
NOBr Lewis structure octet rule
Atoms tend to have eight electrons in their valence shell by sharing of electrons or transfer of electrons between atoms. This is what we call the octet rule.
All the atoms in the Lewis structure of NOBr obeys the octet rule. The Lewis structure of NOBr consists of N, O and Br atoms with each atom having 8 electrons in their valence shell.
Let us discuss octet rule for NOBr here.
- The central Nitrogen atom forms two bonds with oxygen atom and a single bond with Bromine atom. It has a lone pair on it.
- The Oxygen atom has two lone pairs present on it. It forms two bonds with the central Nitrogen atom.
- The Bromine atom has three lone pairs present on it. It forms a one bond with the central Nitrogen atom.
NOBr Lewis structure lone pairs
Lone pairs are the non-bonding electron pair present in the outermost shell of an atom. Let us discuss for NOBr here.
There are 6 lone pairs present in the Lewis structure of NOBr. There are two lone pairs on Oxygen, Br has 3 lone pairs and center atom Nitrogen has one lone pair.
The total number of lone pairs in the lewis structure of NOBr are as follows –
- The number of lone pairs on Nitrogen =1
- The number of lone pairs present on Oxygen = 2
- The number of lone pairs present on Bromine = 3
- Thus, Total lone pairs in NOBr = 6 lone pairs
NOBr valence electrons
Valence electrons are the electrons which decide the combining capacity of an atom. Let us find out valence electrons in NOBr.
The Lewis structure of NOBr consists of 18 electrons. Nitrogen, Oxygen and Bromine belongs to group 15th, 16th and 17th group of the periodic table. N, O and Br have 5, 6, 7 valence electrons in their outermost shell.
The total valence electrons in the NOBr Lewis structure are as follows –
- Nitrogen = 5
- Oxygen = 6
- Central atom Bromine = 7
- Thus, Total valence electrons in NOBr = 5 + 6 + 7 = 18 electrons
NOBr hybridization
Hybridization is the concept according to which atomic orbitals mix to form new hybridized orbitals with equivalent energies. Let us discuss hybridization for NOBr below.
The hybridization of central atom Nitrogen of NOBr is sp2. Nitrogen is bonded to Bromine by a single bond and to Oxygen by a double bond. It has one lone pair on it. This accounts for the sp2 hybridization.
Properties of NOBr
Properties are the characteristics of a substance which we can measure. Let us discuss some physical properties of NOBr here.
| Molecular Weight | 109.91 g/mol |
| Molecular Density | 2.4 g/cm3 |
| Boiling Point | 14.5 degree Celsius (287.6 K) |
| Melting Point | -56.0 degrees celsius |
NOBr soluble in water?
Solubility tells about the ability of the substance to be dissolved in a given solvent especially water. Let us discuss it.
NOBr is soluble in water because it is polar molecule. Water is also polar solvent. NOBr forms hydrogen bonds with water molecules.
Is NOBr solid or gas?
The physical states solid, liquid and gas of a substance is determined by force of attraction between its particles, interparticle space and the kinetic energy of particles. Let us explore.
NOBr is a gas, not a solid point. It is reddish coloured gas The boiling point of NOBr is 14.5 degree celsius.
Why and how is NOBr not a solid?
NOBr is not a solid, it exists as gas because it is formed by the reaction between two gaseous reactant that is Nitric oxide and Bromine. The boiling point of nitrosyl bromide is also low which contributes to its gaseous state.
Is NOBr polar or non-polar?
A polar molecule consists of positive and negative poles while the non-polar molecule does not. Let us disuss for NOBr here.
NOBr is a polar molecule. Due to the lone pair present on the central atom, molecular geometry of NOBr is Bent. The bent shape of the molecule gives it an asymmetric geometry. This makes NOBr a polar molecule.
Why is NOBr polar?
NOBr is a polar molecule because NOBr is a bent shaped molecule which again contributes to the polar nature of NOBr.
Is NOBr acid or base?
As per Bronsted-Lowry theory, Acidic and basic nature depends on the capability to donate and accept a proton. Let as discuss it.
NOBr is a Bronsted-Lowry acid because it can donate a proton to a Bronsted- Lowry base or to an electron rich species which can readily accepts it.
Why is NOBr acid?
NOBr is a Bronsted-Lowry acid because it is formed by the reaction of nitric oxide (neutral in nature) with Bromine (acidic in nature). The pH of Bromine is 4.0. The reaction can be represented as 2NO + Br2 = 2NOBr.
Is NOBr ionic or covalent?
Covalent and Ionic nature is decided by the sharing or transfer of electron(s) respectively. Let us explore it.
NOBr is a covalent compound. It contains one covalent bond between Nitrogen and Bromine and two covalent bonds between Nitrogen and Oxygen.
How and why is NOBr ionic or covalent?
NOBr is ionic because it contains N, Br and O which all are non-metals. Ionic compound must contain a metal. Moreover, the bonds between N and O and that between N and Br are formed by sharing of electron pair(s).
Conclusion:
NOBr is a reddish coloured gas. It has a bent or angular molecular geometry and trigonal planar as electron geometry.
Also Read:
- Formaldehyde lewis structure
- Mgf2 lewis structure
- N2f4 lewis structure
- No lewis structure
- Cno lewis structure
- N2o lewis structure
- Ch3o lewis structure
- Fe2o3 lewis structure
- Caf2 lewis structure
- Pbr3 lewis structure

Hi…I am Sonali Jham. I have done my Post-Graduation in Chemistry and also completed B. Ed. I am a Teacher and a Dietician by profession.
My hobby is reading and painting.
Let’s connect through LinkedIn-