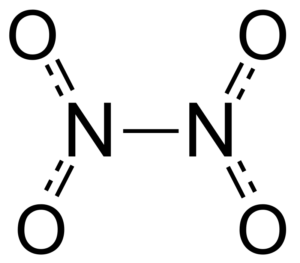
N2O4 Lewis Structure & Characteristics (13 Complete Facts)
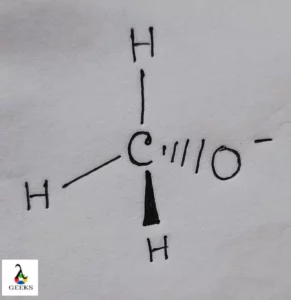
N2O4 Lewis structure refers the formation of bonds between participation Nitrogen (N) and Oxygen (O) atoms in Dinitrogen tetroxide (N2O4). Let us define its Lewis structure below. N2O4 Lewis structure can be described by the method of structuration given by scientist Lewis. This structure denotes the electronic reaction between the N and O atoms of … Read more