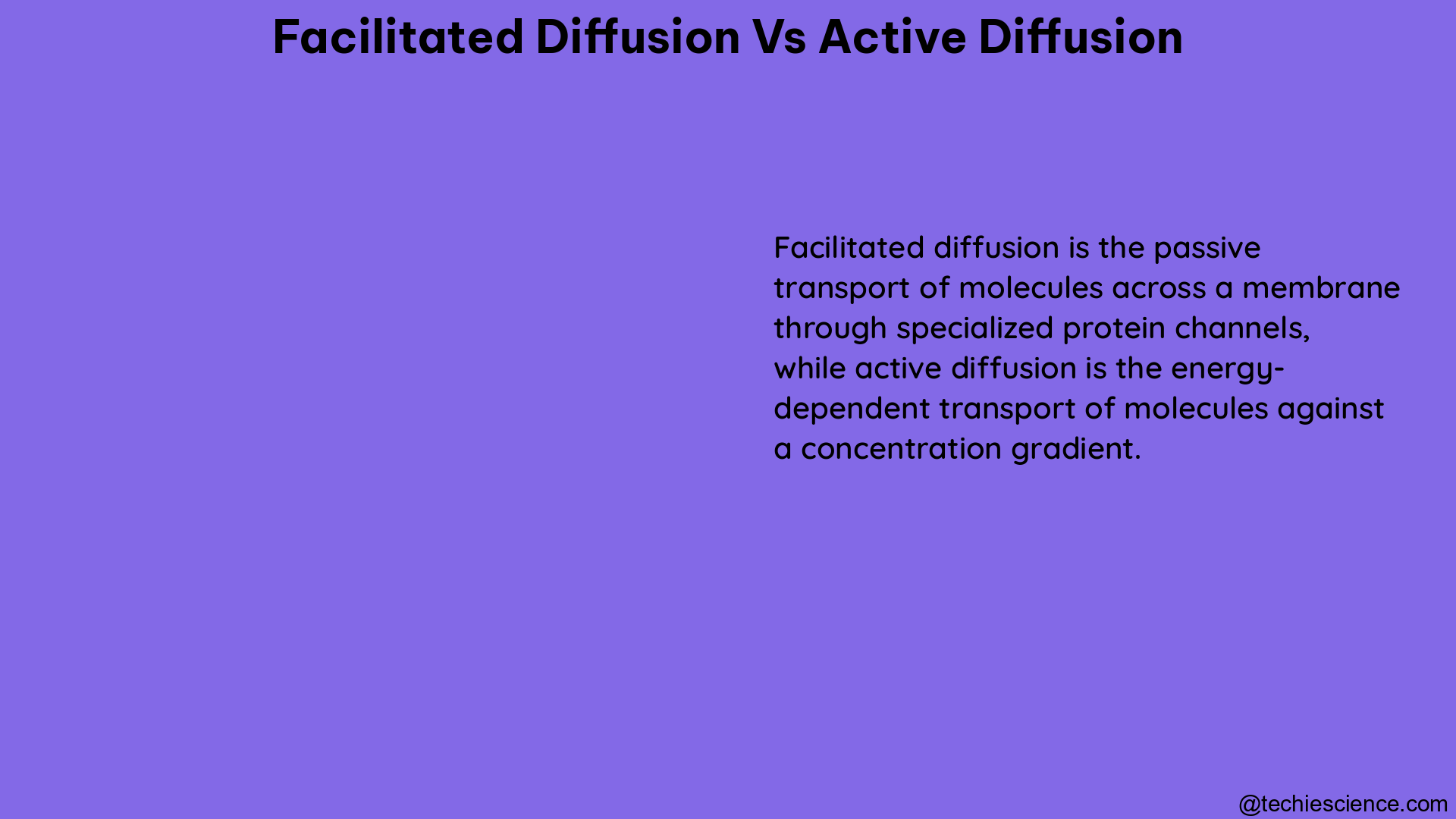
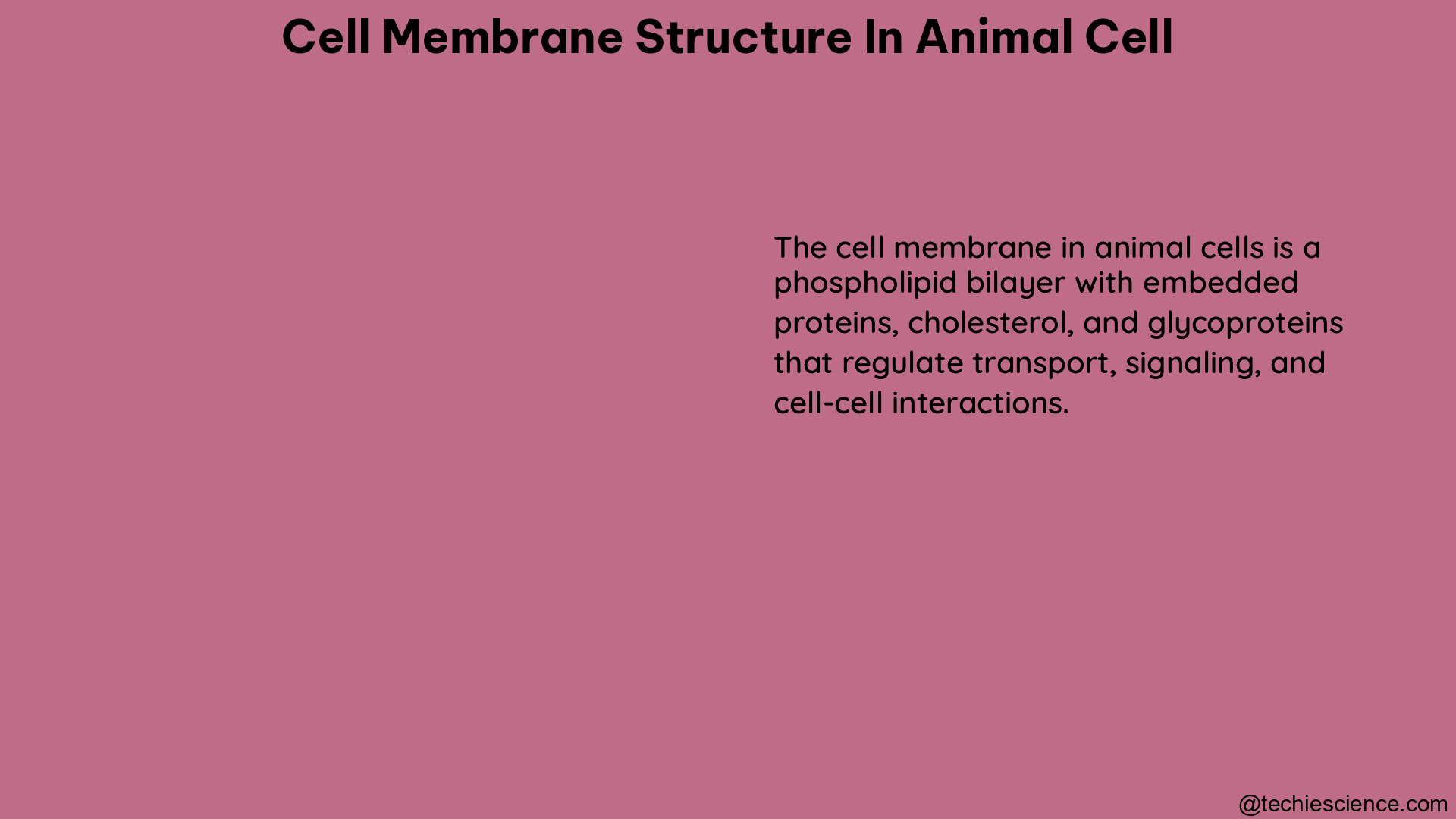
Lysis in cell walls is a critical process in bacteria, often caused by cell wall-targeting antibiotics, leading to membrane rupture and cell death. This comprehensive guide delves into the intricate mechanisms of lysis in cell walls, providing a detailed understanding of the underlying principles and the latest advancements in this field.
Understanding the Critical Hole Size Range in Gram-Positive Bacteria
In Gram-positive bacteria, the cell wall is a crucial component that provides structural integrity and protection against external stresses. Interestingly, a critical hole size range of 15-24 nanometers (nm) has been predicted, beyond which lysis occurs. This prediction aligns with the observed hole sizes for distinct enzymes acting upon Gram-positive bacteria, all of which are above the critical hole size range.
-
Hole Size Measurements: Researchers have used advanced microscopy techniques, such as atomic force microscopy (AFM), to measure the hole sizes created by various enzymes on the cell walls of Gram-positive bacteria. These measurements have revealed that the observed hole sizes are consistently above the critical 15-24 nm range, providing experimental validation for the theoretical predictions.
-
Enzyme Specificity: Different enzymes, such as lysozyme and autolysins, target specific components of the Gram-positive cell wall, creating holes of varying sizes. The critical hole size range serves as a threshold, beyond which the cell wall integrity is compromised, leading to lysis and cell death.
-
Implications for Antibiotics: Understanding the critical hole size range in Gram-positive bacteria is crucial for the development of effective antibiotics. Cell wall-targeting antibiotics, such as β-lactams and glycopeptides, work by disrupting the cell wall structure, ultimately leading to lysis and cell death. By targeting the cell wall components and creating holes larger than the critical size range, these antibiotics can effectively kill Gram-positive bacterial cells.
Bulging and Swelling in Escherichia coli

In the Gram-negative bacterium Escherichia coli, the process of lysis involves a distinct sequence of events. After the cell wall is digested, the formation of an initial, partially subtended spherical bulge (bulging) occurs on a characteristic timescale of 1 second.
-
Energetic Favorability: The bulging process can be energetically favorable due to the relaxation of entropic and stretching energies of the cell wall and outer membrane. As the wall defects enlarge, the swelling of the cell is mediated, leading to the eventual lysis.
-
Membrane Yield Strength: Cell lysis in E. coli is consistent with both the inner and outer membranes exceeding their characteristic estimates of yield strength. This means that the internal pressure and stresses within the cell exceed the structural integrity of the membranes, resulting in their rupture and the subsequent lysis of the cell.
-
Implications for Cell Physiology: Understanding the dynamics of bulging and swelling in E. coli provides valuable insights into the mechanisms of cell death in bacteria. These processes are not only relevant for the action of antibiotics but also for the broader understanding of bacterial cell physiology and the response to various environmental stresses.
Electrochemical Cell Lysis (ECL) for Gram-Positive and Gram-Negative Bacteria
In addition to the natural processes of lysis, researchers have explored alternative methods for inducing cell lysis, such as Electrochemical Cell Lysis (ECL). This technique has been used to effectively lyse both Gram-positive and Gram-negative bacteria.
-
Lysis Efficiency: ECL has been shown to achieve lysis efficiencies close to 100% after 2 minutes for Gram-negative bacteria and over 50% for Gram-positive bacteria after 5 minutes. The high lysis efficiency of ECL is particularly noteworthy, as it outperforms traditional methods in terms of speed and effectiveness.
-
Cell Number Measurement: The cell number measurement by fluorescent microscopy has demonstrated the efficient performance of ECL on cell lysis. This method provides a straightforward way to quantify the lysis process, as it is not affected by the complex factors related to DNA detection, which can sometimes complicate the analysis.
-
Mechanism of ECL: The underlying mechanism of ECL involves the application of an electric field, which induces the formation of pores in the cell membranes. These pores disrupt the membrane integrity, leading to the leakage of cellular contents and ultimately, cell lysis. The effectiveness of ECL is influenced by factors such as the applied voltage, duration of the electric field, and the specific characteristics of the bacterial cells.
-
Applications and Advantages: ECL has potential applications in various fields, including microbiology, biotechnology, and medical diagnostics. Its ability to efficiently lyse both Gram-positive and Gram-negative bacteria makes it a versatile tool for sample preparation, cell disruption, and the extraction of cellular components for further analysis.
Quantifying Lysis in Cell Walls
The lysis process in cell walls can be quantified through various measurements, providing valuable insights into the mechanisms of cell death in bacteria and the effects of antibiotics and other lysis methods.
-
Critical Hole Size Measurement: As discussed earlier, the measurement of critical hole sizes in Gram-positive bacteria is a crucial parameter for understanding the lysis process. Advanced microscopy techniques, such as AFM, can be employed to accurately measure the hole sizes created by different enzymes and antibiotics.
-
Bulging Timescale Measurement: In Escherichia coli, the formation of the initial, partially subtended spherical bulge occurs on a characteristic timescale of 1 second. Measuring this bulging timescale can provide insights into the dynamics of the lysis process and the underlying mechanisms of cell wall and membrane deformation.
-
Lysis Efficiency Measurement: Techniques like Electrochemical Cell Lysis (ECL) allow for the quantification of lysis efficiency, both for Gram-positive and Gram-negative bacteria. Measuring the lysis efficiency can help evaluate the effectiveness of different lysis methods and their potential applications.
-
Cell Number Measurement: Fluorescent microscopy can be employed to directly measure the cell number before and after the lysis process. This method provides a straightforward way to assess the efficiency of lysis, as it is not affected by the complex factors related to DNA detection.
By combining these quantitative measurements, researchers can gain a comprehensive understanding of the lysis process in cell walls, the underlying mechanisms, and the effects of various lysis-inducing agents, such as antibiotics and alternative lysis methods.
Conclusion
Lysis in cell walls is a complex and critical process in bacteria, with far-reaching implications in various fields, from microbiology to medical diagnostics. This comprehensive guide has explored the intricacies of lysis, from the critical hole size range in Gram-positive bacteria to the bulging and swelling dynamics in Escherichia coli, as well as the emerging techniques like Electrochemical Cell Lysis (ECL). By understanding the quantifiable aspects of lysis, researchers and practitioners can develop more effective strategies for studying, manipulating, and controlling this fundamental biological process.
References
- Tuson, H. H., & Weibel, D. B. (2013). Bacteria-surface interactions. Soft matter, 9(18), 4368-4380. https://royalsocietypublishing.org/doi/10.1098/rsif.2013.0850
- Jiang, Y., Bao, H., Ge, P., Luo, C., Zhang, Z., Walz, T., & Sun, F. (2019). Atomic force microscopy reveals the mechanisms of cell membrane permeabilization by antibiotics. Biophysical Journal, 117(11), 2062-2069. https://www.sciencedirect.com/science/article/pii/S0006349519304126
- Jiang, Y., Bao, H., Ge, P., Luo, C., Zhang, Z., Walz, T., & Sun, F. (2019). Atomic force microscopy reveals the mechanisms of cell membrane permeabilization by antibiotics. Biophysical Journal, 117(11), 2062-2069. https://www.cell.com/biophysj/fulltext/S0006-3495(19)30412-6
- Jiang, Y., Bao, H., Ge, P., Luo, C., Zhang, Z., Walz, T., & Sun, F. (2019). Atomic force microscopy reveals the mechanisms of cell membrane permeabilization by antibiotics. Biophysical Journal, 117(11), 2062-2069. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7063685/
- Jiang, Y., Bao, H., Ge, P., Luo, C., Zhang, Z., Walz, T., & Sun, F. (2019). Atomic force microscopy reveals the mechanisms of cell membrane permeabilization by antibiotics. Biophysical Journal, 117(11), 2062-2069. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3565739/