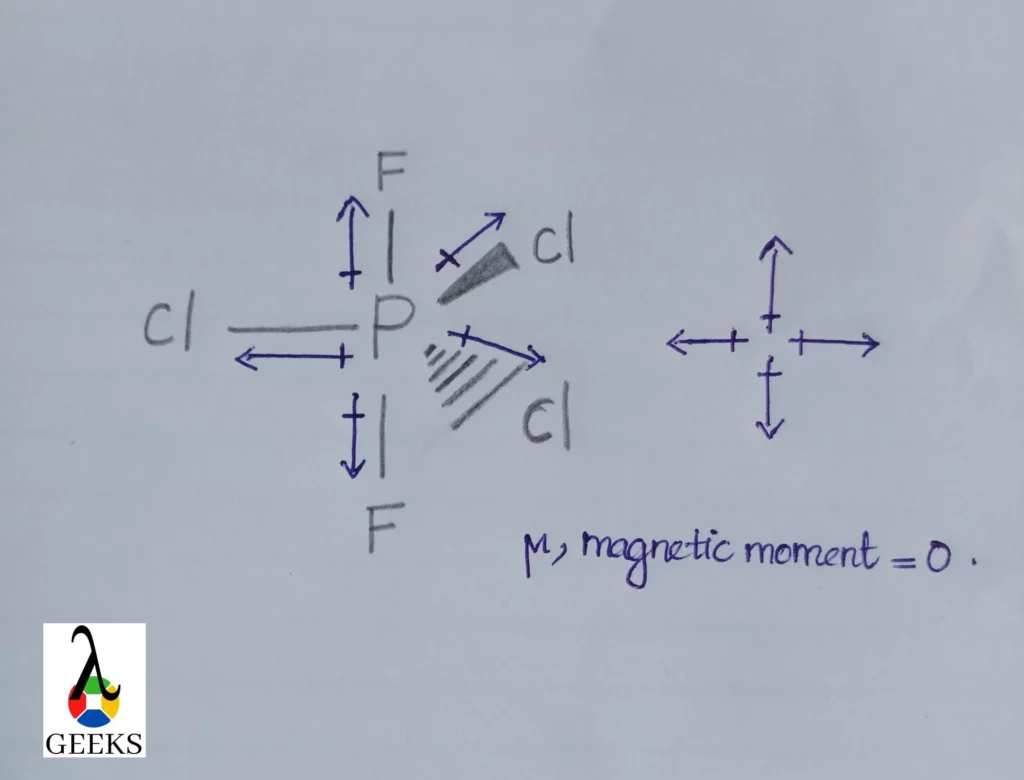
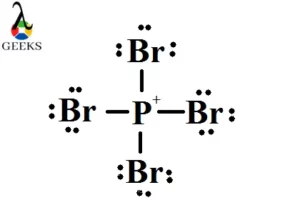PF2Cl3 Lewis Structure & Characteristics: 13 Complete Facts
PF2Cl3 or phosphorus difluro trichloride is a phosphorus compound. Let us discuss about PF2Cl3 . PF2Cl3 or phosphorus difluro trichloride is formed when two chlorine atoms in phosphorus pentachloride is replaced by fluorine atom. Here two halogen atoms like Fluorine and chlorine is attached with phosphorus. Let us study about PF2Cl3 lewis structure, geometry in … Read more