Aluminium belongs to group 13 of the periodic table. Let us have a look at the electronic configuration of aluminium in this article.
Aluminium is one of the coinage metals which is naturally present in the earth’s crust. It is never present in the elemental state. Aluminium is present in the P-block of the periodic table. It exists in a face-centered cubic crystal structure. The aluminium ion has a high charge density, with three positive charges.
We will discuss some facts about the electronic configuration of aluminium such as writing aluminium electronic configuration and orbital diagram of ground state aluminium.
How to Write Aluminium Electron Configuration?
The Al atom consists of 13 electrons. Following Aufbau’s principle electrons will be filled in order of their increasing energies. After that according to Hund’s rule, pairing of electrons takes place in opposite direction as per Pauli exclusion principle.
Aluminium Electron Configuration Diagram
The aluminium atom contains 13 electrons. The electronic configuration of the aluminium is expressed in the form of the diagram as given below-
- 1s orbital having minimum energy is filled first, with a maximum capacity of two electrons.
- After 1s orbital, the 2s orbital is filled with a maximum capacity of two electrons.
- After 2s orbital, the 2p orbital is filled with a maximum capacity of six electrons.
- After 2p orbital, the 3s orbital is filled with a maximum capacity of two electrons.
- After 3s orbital, the 3p orbital is filled with one electron only.
So, the diagram will be-

Aluminium Electron Configuration Notation
The aluminium electronic configuration notation is depicted as –
[Ne] 3s23p1
The aluminium electronic configuration notation consists of a total of 13 electrons out of which two electrons 10 electrons are taken from the Neon gas configuration, two electrons in 3s and one electron in 3p.
Aluminium Unabbreviated Electron Configuration
The electronic configuration for aluminium is depicted as –
1s2 2s22p6 3s23p1
The aluminium unabbreviated electron configuration consists of a total of 13 electrons which are filled as follows –
- Two electrons are present in 1s orbital.
- Two electrons in 2s orbital.
- Six electrons in 2p orbital.
- Two electrons in 3s orbital.
- One electron in 3p orbital.
Ground State Aluminium Electron Configuration
Ground state electron configuration of aluminum is 1s2 2s22p6 3s23p1.
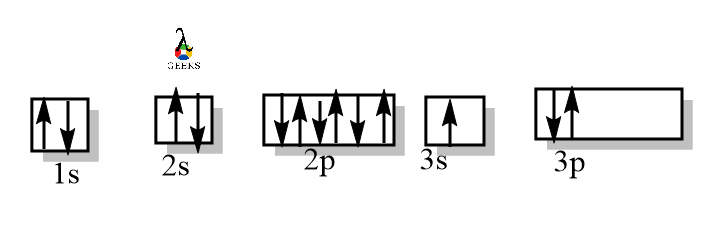
Excited State of Aluminium Electronic Configuration
The excited State of the Aluminium Electronic Configuration is 1s2 2s22p6 3s13p2. The excited state electronic configuration of aluminium is shown below –
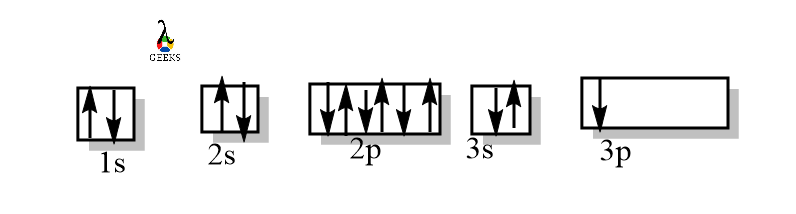
Ground State Aluminium Orbital Diagram
The ground state orbital diagram of the aluminium shows the filling of electrons in the shells around the aluminium nucleus. The orbital diagram of aluminium is-

Aluminium 13 Electron Configuration
The electronic configuration of aluminium 13 is [Ne] 3s23p1. The electrons are filled in various orbitals while considering Aufbau’s principle, Hund’s rule and Pauli’s exclusion principle as given below-
Aluminium Oxide Electron Configuration
The aluminium oxide (Al2O3) electron configuration is as follows-
- The electronic configuration of Al3+ is 1s2 2s22p6.
- The electronic configuration of O2- is 1s2 2s22p2.
Aluminium Condensed Electron Configuration
Aluminium Condensed Electron Configuration is [2, 8, 3].
Aluminium Chloride Electron Configuration
The aluminium chloride (AlCl3) electron configuration is as follows-
- The electronic configuration of Al3+ is 1s2 2s22p6.
- The electronic configuration of Cl– is 1s2 2s22p5.
Conclusion
The electronic configuration of an atom is written in terms of the number of electrons filled in various orbitals having different energy levels. In the case of aluminium, it consists of 13 electrons in its orbitals. Aluminium is used in making steel, automobile parts, household items and many other things.
Also Read:
- Hafnium electron configuration
- Mercury electron configuration
- Cerium electron configuration
- Lithium electron configuration
- Lead electron configuration
- Actinium electron configuration
- Bromine electron configuration
- Copper electron configuration
- Astatine electron configuration
- Technetium electron configuration

Hello everyone, I am Aparna Kushwaha. I have completed my Master’s in Chemistry from the University of Lucknow and doing a Ph.D. in Nanoparticles from the same institute. I have worked as an SME for an ed-tech company for 8 months. Currently, I am working as a Subject Matter Expert with Lambdageeks.