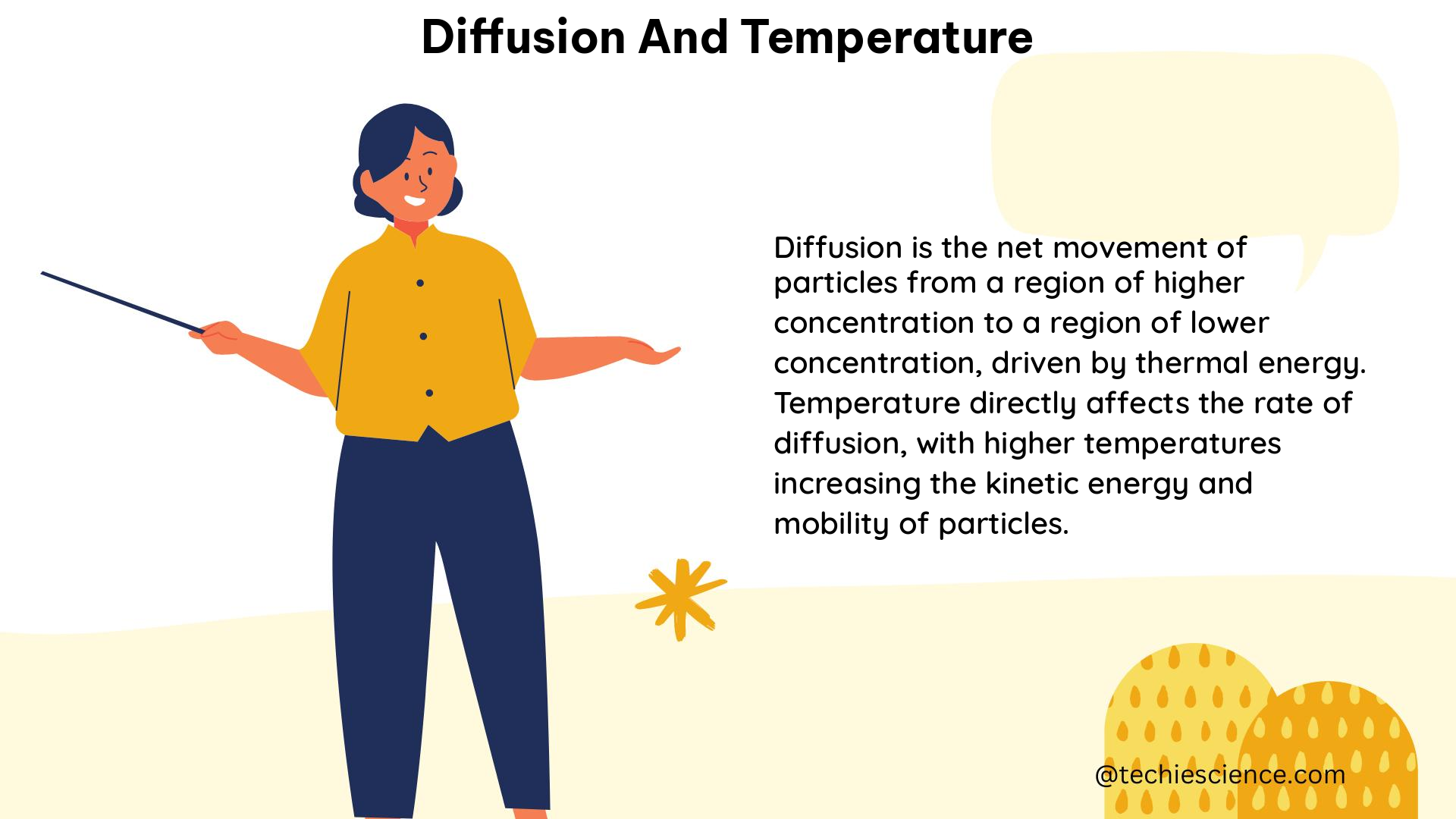
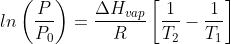Diffusion and temperature are two closely related concepts in physics that play a crucial role in various physical and chemical processes. Understanding the intricate relationship between these two phenomena is essential for students studying physics, materials science, and related fields. This comprehensive guide will delve into the technical details, formulas, and practical applications of diffusion and temperature, providing a valuable resource for physics students.
Diffusion Coefficient: The Key to Understanding Diffusion
The diffusion coefficient is a fundamental parameter that describes the rate at which a substance spreads out in a given medium. This value can be measured in units of area per time, such as square centimeters per second (cm²/s). The diffusion coefficient is directly influenced by temperature, with higher temperatures generally leading to faster diffusion rates.
For example, in a study of diffusion in clay bricks, a diffusion coefficient of 0.014 cm²/s was measured at room temperature. This value is very close to the model prediction for the diffusion coefficient of sample A at an ambient temperature, which is 0.011 cm²/s. The diffusion coefficient can be calculated using the following formula:
D = (kB * T) / (6 * π * η * r)
Where:
– D is the diffusion coefficient (cm²/s)
– kB is the Boltzmann constant (1.38 × 10^-23 J/K)
– T is the absolute temperature (K)
– η is the dynamic viscosity of the medium (Pa·s)
– r is the radius of the diffusing particle (m)
This formula demonstrates the direct relationship between the diffusion coefficient and temperature, as the diffusion coefficient is proportional to the absolute temperature.
Mean Free Path Length and Knudsen Number
In addition to the diffusion coefficient, two other important parameters in the study of diffusion and temperature are the mean free path length (λ) and the Knudsen number (Kn).
The mean free path length is the average distance that a particle will travel before colliding with another particle. It can be calculated using the following formula:
λ = 1 / (√2 * π * d^2 * n)
Where:
– λ is the mean free path length (m)
– d is the diameter of the particles (m)
– n is the number density of the particles (m^-3)
The Knudsen number is the ratio of the mean free path length to the characteristic length scale of the system, and it is a dimensionless quantity. The Knudsen number can be calculated using the following formula:
Kn = λ / L
Where:
– Kn is the Knudsen number (dimensionless)
– λ is the mean free path length (m)
– L is the characteristic length scale of the system (m)
For example, in a study of gas diffusion in clay samples, the mean free path length and Knudsen number were calculated for nitrogen gas at various temperatures, using a mean pore diameter of 0.45 μm. The results showed that the Knudsen number was approximately equal to 1 over the entire temperature range studied, indicating that Knudsen diffusion was the dominant mechanism for gas diffusion in this system.
Temperature Dependency of Diffusion Coefficients
The temperature dependency of diffusion coefficients is often assumed to be exponential, with the diffusion coefficient increasing rapidly with temperature. This relationship can be described by the Arrhenius equation:
D = D0 * exp(-Ea / (R * T))
Where:
– D is the diffusion coefficient (cm²/s)
– D0 is the pre-exponential factor (cm²/s)
– Ea is the activation energy for diffusion (J/mol)
– R is the universal gas constant (8.314 J/mol·K)
– T is the absolute temperature (K)
The Arrhenius equation demonstrates that the diffusion coefficient is exponentially dependent on the inverse of the absolute temperature, with the activation energy (Ea) being a key parameter that determines the temperature sensitivity of the diffusion process.
However, it is important to note that the exact form of the temperature dependency can vary depending on the specific system and conditions being studied. In some cases, the relationship may not be strictly exponential, and other models or empirical correlations may be more appropriate.
Practical Applications of Diffusion and Temperature
The understanding of diffusion and temperature has numerous practical applications in various fields, including:
-
Materials Science: Diffusion plays a crucial role in the processing and properties of materials, such as the diffusion of dopants in semiconductor devices, the diffusion of atoms in metal alloys, and the diffusion of gases in porous materials.
-
Chemical Processes: Diffusion is a fundamental mechanism in many chemical processes, such as the transport of reactants and products in chemical reactions, the separation of gases in industrial processes, and the transport of ions in electrochemical systems.
-
Biological Systems: Diffusion is a vital process in biological systems, governing the transport of nutrients, gases, and other molecules across cell membranes and within living organisms.
-
Energy Storage and Conversion: Diffusion processes are important in energy storage and conversion technologies, such as the transport of ions in batteries and fuel cells, and the diffusion of gases in hydrogen storage materials.
-
Environmental Science: Diffusion plays a role in the transport and fate of pollutants in the environment, such as the diffusion of contaminants in soil and groundwater, and the diffusion of gases in the atmosphere.
By understanding the relationship between diffusion and temperature, students can gain valuable insights into these and other applications, and develop a deeper appreciation for the fundamental principles of physics.
Numerical Examples and Problem-Solving
To further solidify the understanding of diffusion and temperature, let’s consider some numerical examples and problem-solving exercises:
Example 1: Calculating the Diffusion Coefficient
Given:
– Temperature (T) = 298 K
– Dynamic viscosity of the medium (η) = 0.001 Pa·s
– Radius of the diffusing particle (r) = 1 × 10^-9 m
Calculate the diffusion coefficient using the formula:
D = (kB * T) / (6 * π * η * r)
D = (1.38 × 10^-23 J/K * 298 K) / (6 * π * 0.001 Pa·s * 1 × 10^-9 m)
D = 2.59 × 10^-10 m²/s
Example 2: Calculating the Mean Free Path Length and Knudsen Number
Given:
– Particle diameter (d) = 5 × 10^-10 m
– Number density of particles (n) = 1 × 10^28 m^-3
– Characteristic length scale of the system (L) = 1 × 10^-6 m
Calculate the mean free path length (λ) and the Knudsen number (Kn):
λ = 1 / (√2 * π * d^2 * n)
λ = 1 / (√2 * π * (5 × 10^-10 m)^2 * 1 × 10^28 m^-3)
λ = 6.93 × 10^-8 m
Kn = λ / L
Kn = (6.93 × 10^-8 m) / (1 × 10^-6 m)
Kn = 0.0693
These examples demonstrate how to apply the formulas and concepts discussed earlier to calculate the diffusion coefficient, mean free path length, and Knudsen number, which are essential for understanding the behavior of diffusion systems and their relationship with temperature.
Figures and Data Visualization
To further enhance the understanding of diffusion and temperature, it is helpful to incorporate relevant figures and data visualizations. For example, you could include:
-
Graph of Diffusion Coefficient vs. Temperature: This graph would demonstrate the exponential relationship between the diffusion coefficient and temperature, as described by the Arrhenius equation.
-
Plot of Mean Free Path Length vs. Temperature: This plot would show how the mean free path length varies with temperature, which is crucial for understanding the dominant diffusion mechanism in a given system.
-
Contour Plot of Knudsen Number vs. Temperature and Characteristic Length Scale: This plot would provide a visual representation of the Knudsen number as a function of both temperature and the characteristic length scale of the system, helping to identify the regions where Knudsen diffusion is the dominant mechanism.
-
Experimental Data Points and Comparison to Theoretical Models: Incorporating real-world experimental data points and comparing them to theoretical models would provide a more comprehensive understanding of the relationship between diffusion and temperature, and the limitations or assumptions of the models.
By incorporating these types of figures and data visualizations, you can create a more engaging and informative resource for physics students, helping them to better understand the concepts and apply them in practical situations.
Conclusion
In conclusion, the relationship between diffusion and temperature is a fundamental topic in physics that has numerous practical applications. By understanding the key parameters, such as the diffusion coefficient, mean free path length, and Knudsen number, as well as the temperature dependency of these values, students can gain a deeper appreciation for the underlying principles and their real-world implications.
This comprehensive guide has provided a detailed exploration of the technical aspects of diffusion and temperature, including relevant formulas, numerical examples, and data visualization. By mastering these concepts, physics students will be better equipped to tackle complex problems, design experiments, and contribute to the advancement of various fields that rely on the understanding of diffusion and temperature.
References
- Instructables. “Real Diffusion Experiment for Home or School.” Instructables, www.instructables.com/Real-Diffusion-Experiment-for-Home-or-School/.
- CliffsNotes. “Diffusion and Temperature.” CliffsNotes, www.cliffsnotes.com/study-notes/3784767.
- Gao, Yonghui, et al. “Diffusion Coefficient Measurement and Modeling of Methane in Shale Rocks.” Processes, vol. 9, no. 8, 2021, p. 1400., doi:10.3390/pr9081400.
- Cussler, E. L. “Diffusion: Mass Transfer in Fluid Systems.” Cambridge University Press, 1997.
- Incropera, Frank P., et al. “Fundamentals of Heat and Mass Transfer.” John Wiley & Sons, 2007.