Summary
Determining the boiling point of a compound is a crucial step in understanding its physical properties and behavior. This comprehensive guide will provide you with a detailed, hands-on approach to finding the boiling point of a compound using the Thiele tube method. We will delve into the theoretical explanation, advanced practical details, and numerical examples to help you master this essential technique.
Understanding the Boiling Point
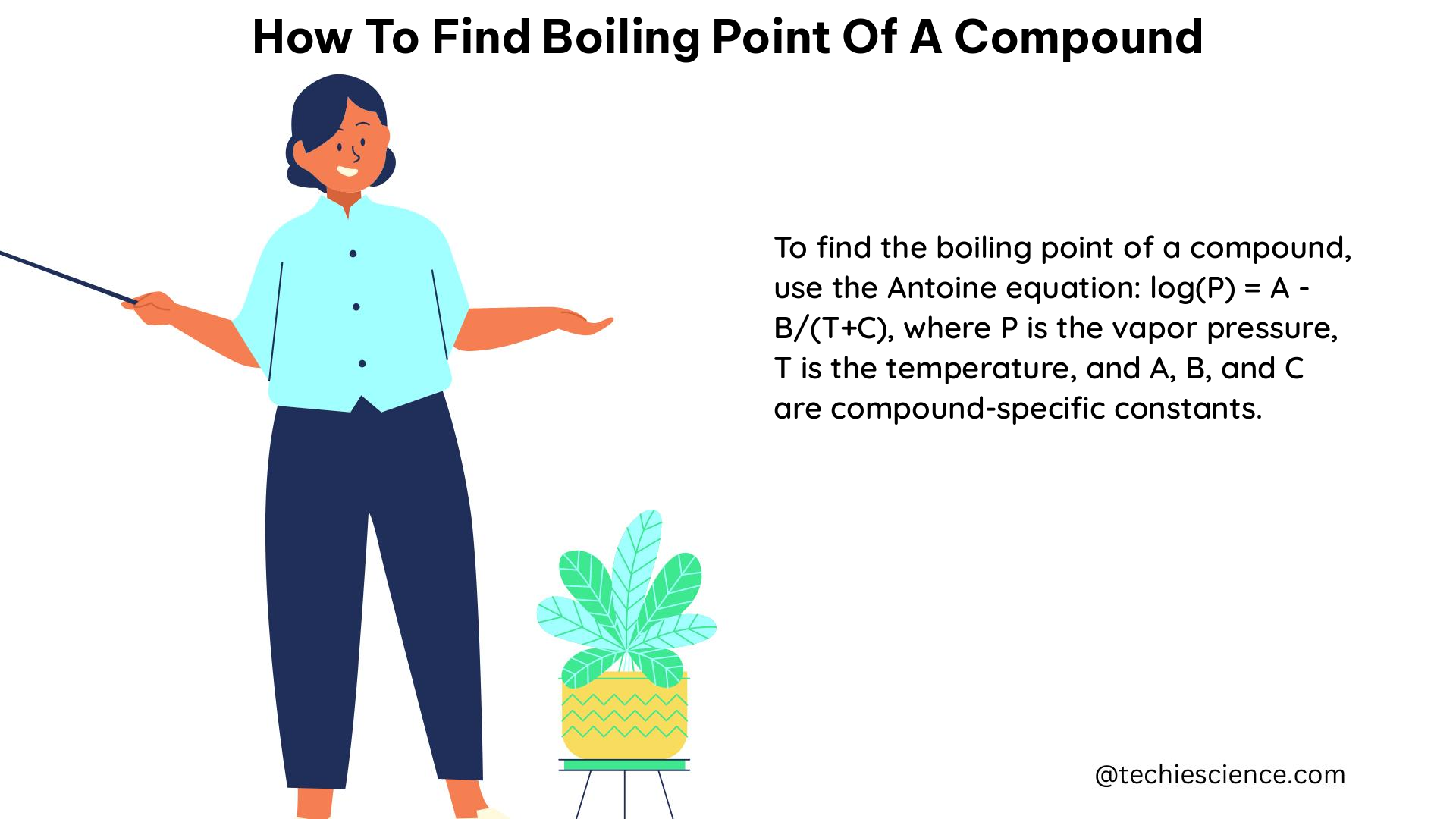
The boiling point of a compound is the temperature at which its vapor pressure equals the surrounding atmospheric pressure. At this point, the compound undergoes a phase transition from a liquid to a gas. The boiling point is a fundamental property that influences various chemical and physical processes, such as distillation, evaporation, and phase equilibria.
Theoretical Explanation
The relationship between the boiling point, vapor pressure, and other thermodynamic variables can be described by the Clausius-Clapeyron equation:
ln(P2/P1) = -ΔHvap/R * (1/T2 - 1/T1)
Where:
– P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively.
– ΔHvap is the molar enthalpy of vaporization (heat of vaporization) of the compound.
– R is the universal gas constant.
By rearranging this equation, we can calculate the boiling point of a compound given the necessary thermodynamic data.
The Thiele Tube Method
The Thiele tube method is a widely used technique for determining the boiling point of a compound due to its simplicity and accuracy. Here’s a step-by-step guide on how to use this method:
-
Prepare the Sample: Fill a small, narrow-necked glass tube (Thiele tube) about half-full with the compound you want to measure the boiling point for.
-
Insert the Capillary Tube: Carefully insert a closed-end capillary tube into the sample-filled Thiele tube, with the closed end facing upwards.
-
Attach the Thermometer: Secure the Thiele tube to a thermometer using a small rubber band or clamp.
-
Heat the Tube: Gently and continuously heat the arm of the Thiele tube using a burner or hot plate.
-
Observe the Bubbles: Continue heating until a vigorous stream of bubbles emerges from the capillary tube.
-
Cool the Tube: Remove the heat source and allow the compound to cool.
-
Determine the Boiling Point: The boiling point is the temperature at which the compound just begins to enter the capillary tube.
This method typically requires less than 0.5 mL of the sample and provides accurate results.
Example: Determining the Boiling Point of Ethanol
Let’s say we want to find the boiling point of ethanol using the Thiele tube method. We follow the steps outlined above and observe that the boiling point of ethanol is 76°C (765 mm Hg).
Numerical Problem
Given the following data, calculate the boiling point of a compound:
P1 = 1 atmT1 = 25°CP2 = 2 atmΔHvap = 40.67 kJ/mol
Using the Clausius-Clapeyron equation, we can solve for the boiling point (T2):
ln(2) = -40670/8.314 * (1/T2 - 1/298.15)
Solving for T2, we get:
T2 = 351.45 K or 78.3°C
Additional Considerations
- The Thiele tube method is suitable for compounds with boiling points ranging from 50°C to 300°C.
- For compounds with very low or high boiling points, other methods, such as distillation or reflux, may be more appropriate.
- Ensure that the Thiele tube is clean and dry before use to avoid contamination.
- Carefully control the heating rate to prevent superheating or bumping, which can affect the accuracy of the boiling point measurement.
- Consider the purity of the compound, as impurities can influence the boiling point.
- Repeat the measurement several times to ensure consistency and accuracy.
Conclusion
Determining the boiling point of a compound is a fundamental task in chemistry and physics. The Thiele tube method provides a simple, efficient, and accurate way to measure this important property. By understanding the theoretical principles, following the step-by-step practical guidelines, and applying numerical examples, you can become proficient in finding the boiling point of a wide range of compounds.
References
- Chem LibreTexts, “Boiling Point Determination,” [Online]. Available: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_Lab_Techniques_(Nichols)/06%3A_Miscellaneous_Techniques/6.02%3A_Boiling_Point/6.2B%3A_Step-by-Step_Procedures_for_Boiling_Point_Determination.
- Socratic, “How do you calculate boiling point?,” [Online]. Available: https://socratic.org/questions/how-do-you-calculate-boiling-point.
- SlideShare, “Determination of Boiling Point,” [Online]. Available: https://www.slideshare.net/slideshow/determination-of-boiling-point/253872205.