Radioactive isotopes eventually decay, or disintegrate, to harmless materials. Some isotopes decay in hours or even minutes, but others decay very slowly.
Radioactive Isotopes Examples are listed below:
Radioactive isotopes are unstable isotopes of chemical elements which have different atomic mass than defined by the periodic table.
Strontium-90 and cesium-137 have half-lives of about 30 years (half the radioactivity will decay in 30 years). Plutonium-239 has a half-life of 24,000 years.
The unstable radioactive isotopes decay emitting alpha, beta or gamma rays to form the stable nuclei or sometimes another unstable nuclei or radionuclei as it is commonly called. The decay times of a radionuclei vary widely and it is designated by its half-life.
Around 2400 radionuclei have half-lives less than 60 minutes, most of which are produced artificially. A few of the radionuclei have very high half-lives ranging above 100 million years such as Uranium and thorium. They can occur both naturally as well as synthesized artificially.
Radioactive Isotopes Examples Detailed Explanations
Examples of radioactive isotopes and their usage can found in almost all fields of modern science, whether it is medicine, biology, food preservation, mining, industrial applications, astronomy, particle physics etc.
Tritium
The lightest radioactive isotope is of hydrogen, which has a mass number of 3 and called Tritium. It has 2 neutrons in its nucleus and one proton. Tritium is naturally occurring isotope, which means it forms naturally by means of cosmic rays which fall on the nitrogen molecule breaking it to form tritium. Tritium is also formed in nuclear reactors carrying out fission reaction as by products or through various nuclear weapon explosions. It has a half life of around 12.3 years. It is also available in nature in very small amount. Reacting with oxygen it converts into water form and being a part of the food chain.
Carbon-14
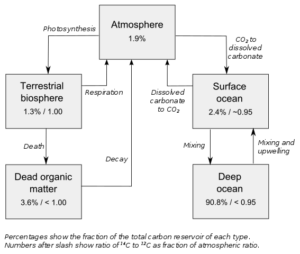
Carbon-14 is the radioactive isotope of carbon having 6 protons and 8 neutrons. By far the most common use of carbon-14 is in archaeological dating. The time of death of an organism can be estimated easily by determining the quantity of carbon-14 available in a dead organism. The interaction of cosmic ray and nitrogen atoms results the formation of carbon-14 naturally in the atmosphere.
Carbon dioxide formed from carbon-14 is absorbed by plants and passed on to the food chain. The carbon-14 in an organism is continuously being replenished till the organism is alive and its quantity starts to reduce by emission of beta rays. It has a half life of around 5700 years.
Cobalt-60
Cobalt 60 is an isotope of cobalt. It doesn’t form naturally can be produced artificially by bombarding a cobalt-59 source by slow neutron source and by nuclear reactor operations. Cobalt-60 decays by emitting gamma rays, with a relatively high intensity. Cobalt-60 when ingested is partly excreted in faeces but some amount of it is also absorbed by kidney and liver which leads to development of cancer cells.
External exposure to high level of gamma radiation emitted by cobalt-60 also causes skin burns, acute radiation sickness and death. It has a half life of 5.3 years.
Iodine-129
Iodine-129 occurs naturally in small quantity but it has gained notoriety because of its formation during nuclear weapon testing and by product of nuclear fission reactors along with its very long half life extending to millions of years.
The long half life of iodine-129 makes it suitable for dating of meteorites and ground water. Iodine is normally absorbed by the thyroid gland and is used to produce hormones. Radioactive iodine is ingested by human, it would be absorbed by thyroid glna d leading to thyroid cancer.
Iodine-131
Like Iodine -129, Iodine-131 too is produced as by product of nuclear fission and nuclear weapon testing. Commercially it is produced from neutron irradiation of naturally occurring tellurium. It has a half life of around 8 days and hence considered less dangerous than iodine-129. Exposure to Iodine-131 has same impacts as that of Iodine-129.
Thorium-232
Thorium-232 is an isotope of thorium element and it has the longest half life among the radionuclei of more than 14 billion years and hence it occurs naturally. It undergoes alpha decay to form radium- 228. Among the isotopes of thorium, thorium-232 is the most abundant.
Thorium-232 can be converted to Th-233 by capturing a neutron which is unstable. Thorium-233 produces fissile isotope uranium-33 by undergoing two consecutive beta decays.
Uranium-235
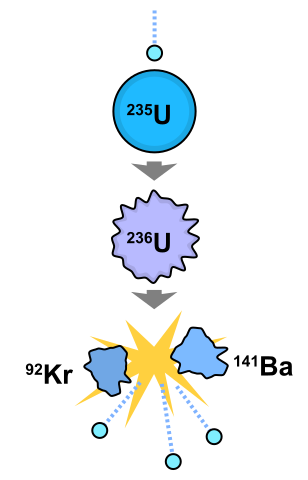
Uranium-235 exits naturally and hence it is called primordial radioactive isotope. The abundance of U-235 in the predominant isotope U-238 is around 0.7%. Uranium-235 is fissile, i.e. it can sustain a nuclear chain reaction and hence it is the predominant fuel in nuclear reactors around the world. It has a half life of around 700 million years.
Plutonium-239
Plutonium is one on the three fissile radioactive isotopes which can be used for both nuclear weapons as well as in nuclear reactors. The other two being uranium-235 and uranium-233.
Among the fissile radioactive isotopes, plutonium-239 has smallest critical mass:- which can be explained as the minimum amount of fissile material to sustain nuclear fission reaction. Plutonium-239 can be synthesized in nuclear reactor from uranium-239 and it has a half life of more than 24000 yea
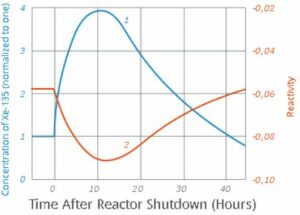
Xenon-135
Xenon-135 isotope is formed in the nuclear reactors in the fission reaction of Uranium-235. It is an unstable isotope having a half life of 9.2 hours. In the U-235 nuclear reactors, it acts a reaction poison by virtue of its neutron absorbing capability.
In fact, Xenon-135 is strongest known neutron absorber and the uranium fission reaction yields around 6% Xenon-135, which temporarily slows down and inhibits the nuclear reaction as it starts building up the core.
The phenomenon of decrease in reactivity Uranium reactor due to build-up of Xenon-135 is called iodine pit. The name iodine is derived from the fact that, in the uranium decay chain, Xenon-135 is formed by beta decay of Iodine-135.
Radon-222
Radon-22 is the isotope of radon gas. It is formed in the decay chain of uranium-238, the most abundant of uranium isotopes. Radon-222 thus occurs naturally. The immediate precursor to Radon-22 in U-238 decay chain in radium-226. It has a half-life of 3.2 days.
Owing to its natural origin and occurrence and being gaseous in nature, the risk of radiation exposure to general public is high for radon-222. It can form in the soil and rock beneath from decay of uranium-238 and permeate through openings and cervices to the buildings above. Long term radiation exposure to Radon-222 causes lung cancer.
Radioactive Isotopes Uses
Radioactive isotopes find use in various fields primarily being Energy, archaeology and medical sciences.
Over the years, radioactivity and radioactive isotopes found use in various fields.The various uses of radioactive substances can be broadly classified as follows:
- Nuclear Energy and Weapons
- Radiocarbon and Surface Exposure Dating
- Medical Use and research
- Industrial Use
Radioactivity as a phenomenon was discovered towards the end of 19th of century; however it attained worldwide attention upon its use in manufacture and use of nuclear weapons during World War II.
Nuclear Energy and Weapons
Radioactive isotopes can produce nuclear energy by means of nuclear fission reaction and those radioisotopes which can sustain a nuclear fission chain reaction are called fissile.
The commonly used radioisotopes for this purpose are Uranium-233, Uranium-235 and Plutonium-239. The energy produced by nuclear fission can be utilised to produce electricity in a nuclear power plant, power naval submarines or manufacture nuclear war heads to be used in missiles.
Radiocarbon and Surface Exposure dating
Radiocarbon dating and exposure dating both dating technologies are utilised to determine the age of an object.This is because, when an organism is alive, its carbon-14 component is in equilibrium with that present in the environment as the organism continuously exchanges carbon though food in case of animals and through photosynthesis in case of plants.
As the organism dies, carbon -14 starts radioactive decay with a half life of around 5700 years. So by measuring the amount of carbon-14 left in a dead tree trunk or a piece of bone, the period when the organism was living can be determined.
Surface exposure dating is utilised to determine the exposure of a rock or a surface to the atmosphere or how long it has been kept buried.
A number of radioactive isotopes such as Beryllium-10, Aluminium-26, Iodine-129, calcium-41 etc are formed by interaction of cosmic rays with its parent isotope. So by measuring the amount of these radioisotopes in rock or water samples, its age can be determined.
Medical Use and research
Radioactive isotopes finds numerable use in medical and biomedical research fields from medical treatment in form of nuclear medicine, diagnosis to study of cellular function and bone formation in animals.
In medical field, Iodine-123 and Iodine -131 are utilised for treatment thyroid disorders, while Iodine-125 and Iodine-129 are used for diagnosis of thyroid disorders. Radioisotopes Cesium-137, Cobalt-60 and Copper-67 are used for treatment of cancer.

Radioisotopes Phosphorus-32 and Phophorus-33 are utilised in molecular biology and genetics research. Others such as Selenium-75 and Strontium-85 are utilised in various studies of life sciences like bone formation, metabolism etc.
Industrial Use
Radioactive isotopes find wide range of industrial use.
Iridium-192 is used to check the weld integrity of pipelines, vessels, aircraft parts etc. Amricum-241 is used in the smoke detectors. Californium-252 is used to check for hidden explosives in luggage at airport.
Radioactive Isotopes Types
Radioactive isotopes can be broadly classified into two types: – naturally occurring and synthetic
Naturally occurring radioactive isotopes are those, which occur naturally and whose traces can be found on the environment and they were not created due to any human activity.Synthetic radioisotopes are those that are formed either as a by product of nuclear fission reaction or synthesized deliberately in nuclear reactors and particle accelerators.
Naturally occurring radioactive isotopes can be further classified to: Primordial, Secondary and Cosmogonic isotopes.
Primordial radioactive isotopes are those that were formed with the formation of universe and their half lives are so large that complete decay to daughter isotopes has not been completed. Hence, they can be found in nature, like the isotopes of Uranium and thorium.
Secondary radioactive isotopes are those, which are formed by radioactive decay of primordial radioactive isotopes. These are intermediate radioactive isotopes in the decay chain of primordial isotopes, e.g. polonium and radium.
Cosmogonic isotopes are those which are formed by the impact of cosmic rays on a stable element. For example radioisotope carbon-14 is formed by impact of cosmic rays on nitrogen.
Synthetic radioisotopes are also produced due to nuclear explosions that were carried out in the past.
Some of the examples of synthesized radioactive isotopes are plutonium-238, plutonium -239, Americium-241, Gadolinium-153 etc.
Radioactive Isotopes Properties
The chemical properties of a radioactive isotope are similar to that of the non-radioactive stable isotope of the same element; however it is unstable because of excess nuclear energy.
The nucleus of a radioactive isotope has an extra neutron and hence has higher atomic mass but having the same number of electrons as that of the stable isotope. Since chemical reaction depends upon the electrons, chemical properties are same for all isotopes of an element.
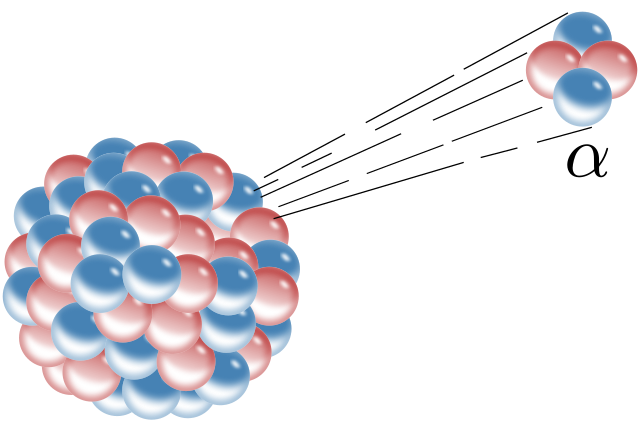
The excess energy of a radioisotope is released by means of alpha, beta or gamma radiation decay. The radiation decay of a radioisotope results in formation of isotope of another element or same element, which can either be a stable or an unstable isotope. If the resultant isotope is unstable, further decay takes place until it becomes a stable element. The simultaneous decay of an unstable radioactive isotope is also called its decay chain.
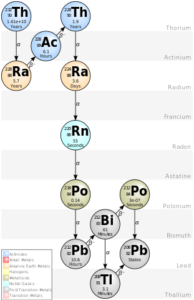
All radioactive isotopes are defined by their half-life or amount of time it requires to decay to 50% of its original mass. The half-life of radioisotopes are specific to a isotope and this property is utilised for various scientific studies raging from age dating of dead organic material to exposure dating of rocks, groundwater etc.
Some radioisotopes have a half life of few seconds to minutes, while other have half life as large as age of universe