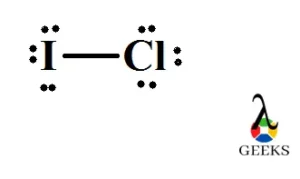ClF2- Lewis Structure & Characteristics: 11 Complete Facts
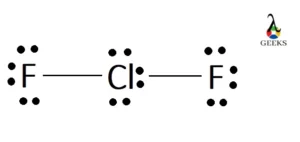
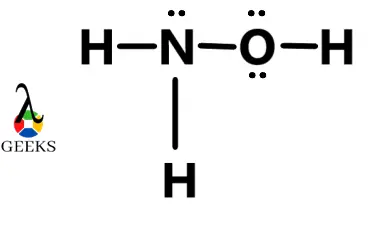
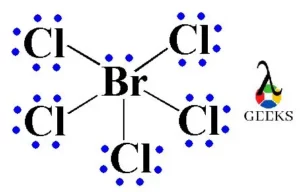
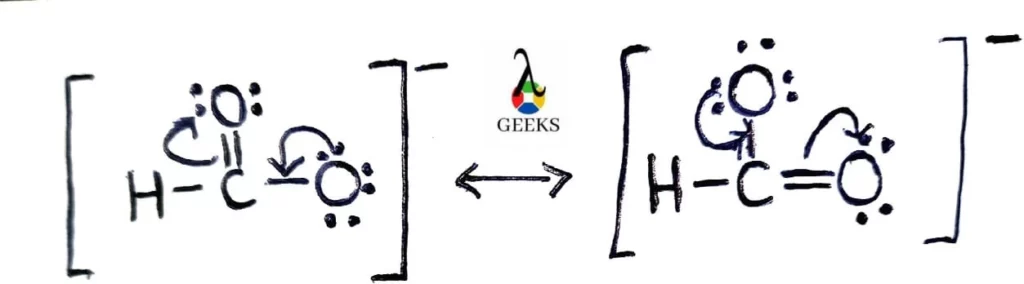
Chlorine difluoride (ClF2) is a chemical compound that consists of one chlorine atom and two fluorine atoms. It is a highly reactive and toxic gas that is primarily used in the production of uranium hexafluoride for nuclear fuel. The Lewis structure of ClF2 helps us understand the arrangement of atoms and the distribution of electrons … Read more