PH5 Lewis Structure & Characteristics: 9 Complete Facts
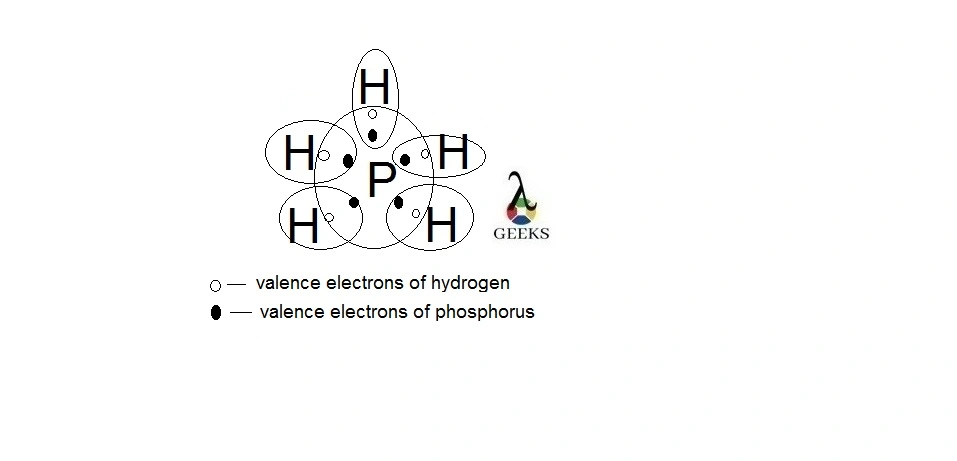
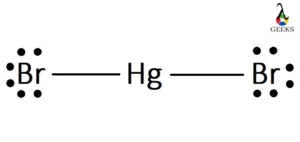
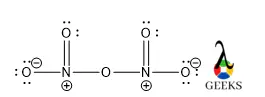
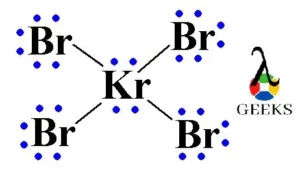
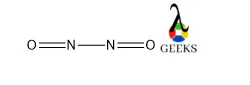
Phosphorane or PH5 is a very unstable chemical compound. It is a compound of pentavalent phosphorus. Let us talk about characteristics of PH5 in details below. PH5 is a hypothetical molecule. The structure adopts trigonal bipyramidal geometry with two longer axial bonds and three comparatively short equatorial bonds. This is because of the more electron … Read more