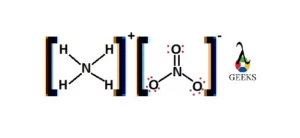
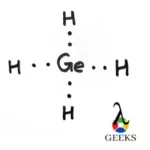
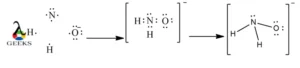
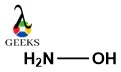Explore the world of molecular diagrams with our comprehensive guide on Lewis structures. Learn about the fundamental concepts, historical background, and key applications in an easy-to-understand format. Perfect for students and chemistry enthusiasts, this guide offers insights into chemical bonding and molecular structure, making it an invaluable resource for anyone looking to enhance their knowledge in chemistry
Introduction to Lewis Structures
Understanding the Basics
Lewis structures, also known as Lewis dot diagrams, are a way to represent molecules showing how atoms are bonded together and where the valence electrons are distributed. These simple diagrams are a foundational concept in chemistry, especially useful for students and professionals across the United States.
Why Lewis Structures Matter
These structures are more than just drawings; they provide insights into the behavior of molecules, including their reactivity, polarity, and the formation of chemical bonds. They are essential tools in both educational settings and professional chemical research.
Historical Background of Lewis Structures
The Origins
The concept of Lewis structures originated from the work of Gilbert N. Lewis, an American chemist, who introduced them in 1916. His groundbreaking paper, “The Atom and the Molecule,” laid the foundation for what we now understand as the covalent bond.
Gilbert N. Lewis’s Contributions
Lewis’s ideas revolutionized the way chemists understand molecular structure. By proposing that atoms combine by sharing electrons, he provided a visual and practical way to represent molecules, paving the way for modern chemical bonding theories.
Key Concepts in Lewis Structures
Understanding Electrons and Valence Shells
The core idea behind Lewis structures is the representation of valence electrons (the outermost electrons) in atoms. These electrons play a crucial role in bonding and chemical reactions.
Rules for Drawing Lewis Structures
- Identify Valence Electrons: Begin by determining the total number of valence electrons for each atom in the molecule, which are crucial for bonding.
- Arrange Atoms: Place the least electronegative atom in the center (usually carbon in organic molecules) and surround it with other atoms.
- Distribute Electrons to Fulfill Octet Rule: Electrons are shared or assigned to give each atom, especially the central atom, a complete octet (8 electrons). Hydrogen is an exception, requiring only 2 electrons.
- Double and Triple Bonds for Additional Stability: When single bonds don’t fulfill the octet rule for all atoms, introduce double or triple bonds. These bonds are often formed with elements like oxygen, nitrogen, and carbon to achieve stability.
- Check Formal Charges: Ensure that the structure with the least formal charges, or the most stable arrangement of charges, is chosen as the preferred Lewis structure.
These rules provide a systematic approach to drawing Lewis structures, helping to visualize molecular structure and bonding.
How to draw Lewis Dot structure
When it comes to drawing Lewis Dot structures, following a systematic process ensures accuracy and clarity in representing molecular bonding and electron arrangements. Here are the detailed steps to guide you through this process:
Step 1: Determine the Total Number of Valence Electrons
- Explanation: The first step in drawing a Lewis Dot structure is to determine the total number of valence electrons available in the molecule. Add up the valence electrons from each atom in the molecule. Remember, valence electrons are the electrons in the outermost shell of an atom and are involved in chemical bonding.
- Example: In carbon dioxide (CO2), carbon has four valence electrons, and each oxygen atom has six. The total number of valence electrons is (4 + 6 times 2 = 16).
Step 2: Choose the Central Atom
- Explanation: The central atom is usually the least electronegative element that isn’t hydrogen. It’s the atom around which other atoms are arranged. In many molecules, this is a single atom.
- Example: In CO2, carbon is less electronegative than oxygen and is not hydrogen, so carbon is the central atom.
Step 3: Sketch a Skeleton Structure
- Explanation: Connect the central atom to the surrounding atoms using single bonds. At this stage, you’re setting up a basic framework for the molecule.
- Example: For CO2, place the carbon atom in the center and draw single bonds to each oxygen atom on either side.
Step 4: Distribute the Valence Electrons
- Explanation: Starting with the outer atoms, place the remaining valence electrons to satisfy each atom’s need for an octet (eight electrons). Distribute the electrons as lone pairs around the atoms.
- Example: In CO2, after forming two single bonds, 12 valence electrons remain. Distribute these around the oxygen atoms, giving each oxygen six additional electrons to complete their octets.
Step 5: Convert Lone Electron Pairs to Bonds if Necessary
- Explanation: If the central atom does not have a complete octet, form double or triple bonds by converting lone pairs on the surrounding atoms into shared electron pairs (bonds).
- Example: In CO2, carbon has only four electrons after step 3. Convert a pair of lone electrons on each oxygen into additional shared bonding pairs (double bonds) with carbon to complete the octet for carbon.
Step 6: Verify Each Atom Satisfies the Octet Rule
- Explanation: Check that each atom (except hydrogen) has eight electrons around it. This can include both the electrons in the shared bonds and the unshared lone pairs.
- Example: In the final structure of CO2, each oxygen has eight electrons (two from the double bond with carbon and six as lone pairs), and carbon has eight electrons shared in double bonds with the two oxygens.
Each step is crucial for accurately drawing Lewis Dot structures. This systematic approach ensures that the molecular structure adheres to the rules of chemical bonding and electron distribution.
Common Examples and Variations of Lewis Structures
Simple Molecules
- Water (H2O): Showcasing a classic bent shape with two pairs of nonbonding electrons on the oxygen atom.
- Carbon Dioxide (CO2): A linear molecule with double bonds between the carbon and oxygen atoms.

Complex Molecules
- Organic Compounds: Such as methane (CH4) and ethylene (C2H4), illustrating how Lewis structures can represent larger, more complex organic molecules.
Variations in Lewis Structures
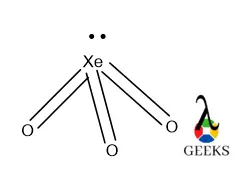
- Resonance Structures: Some molecules, like ozone (O3), have multiple valid Lewis structures, known as resonance structures.
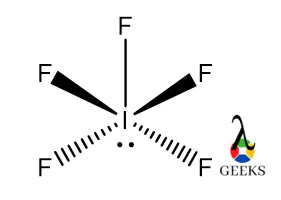
- Exceptions to the Octet Rule: Elements in the third period and beyond can have expanded octets, as seen in molecules like sulfur hexafluoride (SF6).
Application in Various Fields
Chemistry Education
- Lewis structures are fundamental in chemistry education, helping students in the USA and globally to grasp the basics of molecular structure and bonding.
Research and Industrial Applications
- In research, these structures aid in predicting molecular behavior, crucial for drug design, material science, and environmental studies.
- In industries, understanding Lewis structures helps in developing new compounds and materials, ranging from pharmaceuticals to advanced polymers.
Conclusion and Further Reading
Recap of Key Points
- Lewis structures are a fundamental tool in understanding molecular bonding and structure.
- Originating from the work of Gilbert N. Lewis, these structures have become an essential part of chemistry education and research.
- They are versatile, depicting simple to complex molecules, and are applicable in various scientific and industrial fields.
Linking to Existing Content
- For more detailed examples and explanations, check out our extensive collection of 400 Lewis structure posts, covering a wide range of molecules.
FAQs
- What is a Lewis Structure?
- A diagram representing the arrangement of valence electrons around atoms in a molecule.
- Why are Lewis Structures Important?
- They help visualize molecular structure and predict bonding, reactivity, and properties of molecules.
- How do You Determine the Number of Valence Electrons in a Lewis Structure?
- Count the electrons in the outermost shell of each atom in the molecule.
- What is the Octet Rule in Lewis Structures?
- It’s the principle that atoms tend to bond in a way that each atom has eight electrons in its valence shell.
- Can All Molecules be Represented by a Lewis Structure?
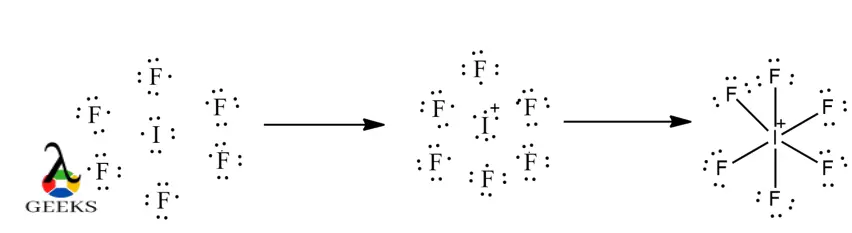
- Most, but not all. Some molecules, especially those involving d-orbitals, may not conform neatly to Lewis structure rules.
- What are Resonance Structures?
- Different possible Lewis Structures for a molecule where electron pairs or bond arrangements vary.
- How are Double and Triple Bonds Represented in Lewis Structures?
- By two or three parallel lines between atoms, representing shared pairs of electrons.
- What Does a Lone Pair in a Lewis Structure Indicate?
- A pair of valence electrons not involved in bonding.
- How Do Formal Charges Relate to Lewis Structures?
- They help determine the most stable structure by revealing electron distribution in the molecule.
- Are Lewis Structures Used for Predicting Molecular Shape?
- Yes, they can provide initial insights, but VSEPR theory is more precise for determining molecular geometry.
Also Read: