Silicon Electron Configuration:7 Easy Steps on How to Write
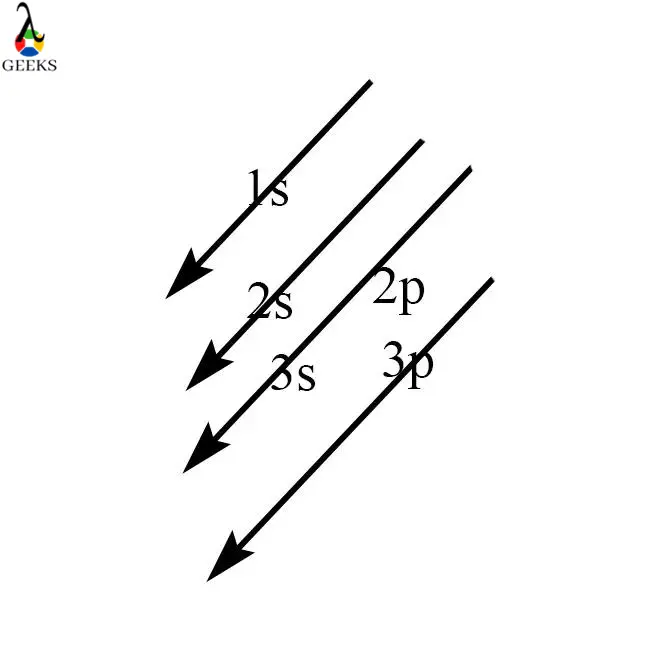
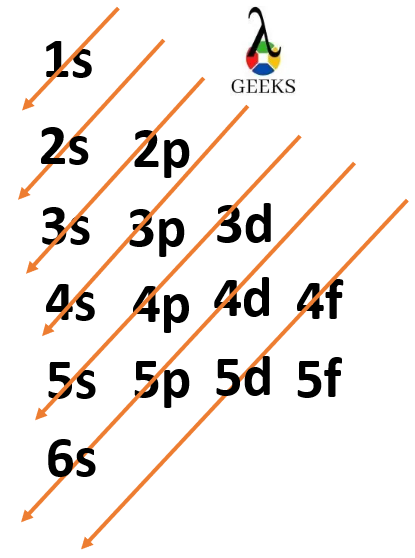
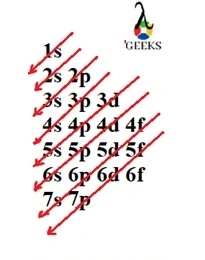
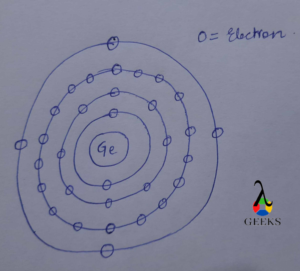
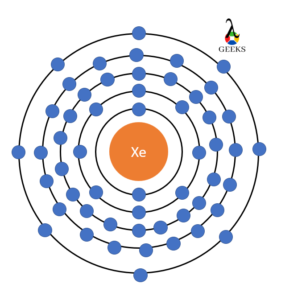
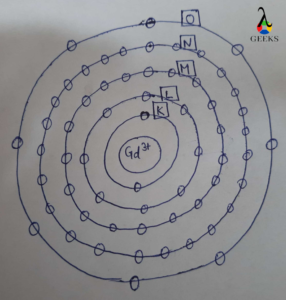
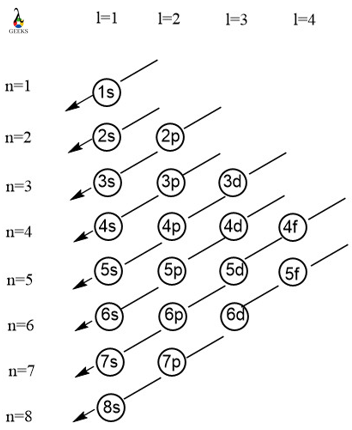
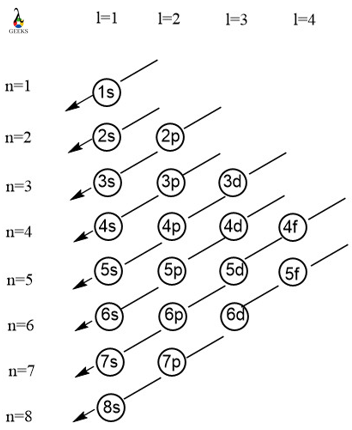
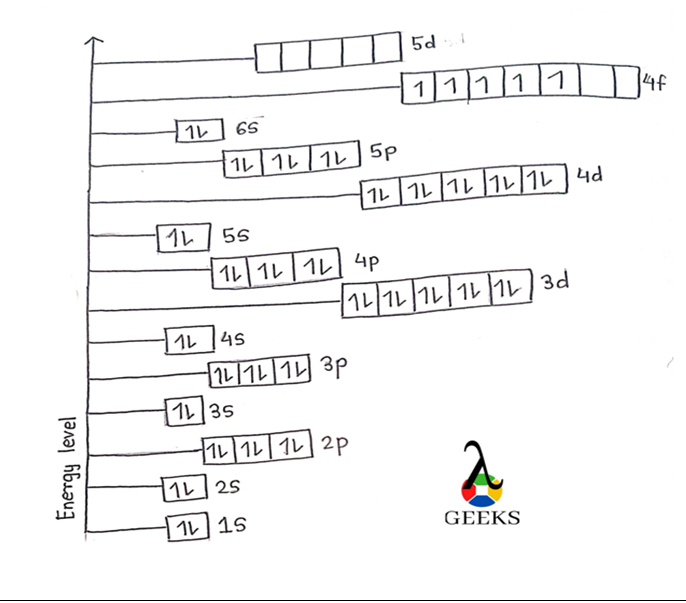
Electronic configuration describes how electrons can be put in an orbit. Let us discuss some facts about the electronic configuration of silicon in the following article. The atomic number of silicon is 14 and its electronic configuration is [Ne] 3s² 3p². Silicon has a hard and crystalline solid-like structure. It belongs to group 14 in … Read more