This article discusses 15+ structural isomers example, their property, classification, and detailed fact.
A structural Isomer is one kind of constitutional isomer. A molecule has the same molecular formula but differs in skeleton structure. Constitutional isomers have different physical properties due to different skeleton frameworks but the same chemical properties, causing their chemical formula same.
structural isomers example
- Butane
- Pentane
- Hexane
- 1,2-dibromobenzene
- Butene
- Pentene
- Cyclohexane
- Cyclopropane
- Cyclobutene
- Ether/Alcohol
- Aldehyde/ketone
- Acid/ester
- Keto-enol
- Dicarbonyl
- Enolate ion
- Phenol
Chain Isomer
Chain isomerism occurs in different arrangements of carbon-carbon chains in a molecule. Chain isomerism will show in that molecule having a carbon-carbon single bond only. The physical property of the two, chain isomer will be different but the chemical property is the same.
Butane
The molecular formula C4H10 has two different structures due to the arrangement of the chain linked with carbon. Former is Butane and the latter is isobutane. Again, their chemical property the same but their physical property is different.

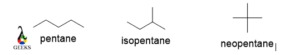
Pentane
The molecular formula C5H12 has three different structures due to the arrangement of single carbon-carbon links. One is n-pentane , and other two are isopentane and neopentane.
Pentane has a higher boiling point due to longer chain size, so the van der Waal attraction force is higher here. For Neopentane the structure is long as compared to n-pentane. So. the boiling point of neopentane is lower. The boiling point of isopentane lies between n-pentane and neopentane. The boiling point of n-pentane, isopentane, and neopentane are 314,313 and 310 K respectively.
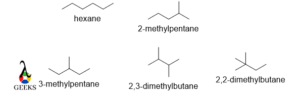
Hexane
n-Hexane has the highest boiling point among these isomers. N-hexane has long-chain conformation, so the surface area is higher and the van der Waal attraction will be high. Between 2-methyl pentane and 3-methyl pentane, later has a higher boiling point. 2,2-dimethyl butane has the least boiling point as it has the lowest surface area and the force of attraction is very low.
The boiling point of n-hexane, 2methylpentane, 3methylpentane, 2,3-dimethyl butane, and 2,2-dimethyl butane are 342, 333, 336,331, and 323 k respectively.
Positional Isomer
Molecules have the same formula but differ in the position of the functional group in the carbon skeleton is called positional isomer.
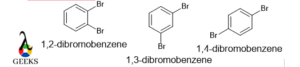
1,2-dibromobenzene
The boiling point of 1,4-bromobenzene is higher than any other isomers. Because this isomer shows molecular symmetry, so in crystallography data is higher.
Butene
The molecular formula of these above compounds is C4H8 but the position of a double bond is different. So, they are called positional isomers. The former has less substituted double bond as compared to the next. The stability of a more substituted double bond is high due to the hyperconjugation effect. So, the reactive center will be different for those molecules.
So, the latter has greater stability than the former.

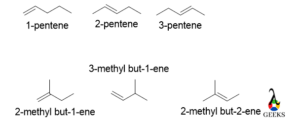
Pentene
All the above molecules have the same molecular formula that is C5H10 but differs in the position of the double bond and the functional group in the main carbon skeleton. For 1-pentene, 2-pentene, and 3-pentene differ from each other by the position of the double bond. But 2-methylbut-1-ene, 3-methylbut-1-ene, and 2-methylbut-2-ene differ from each other by a double bond as well as the functional group also.
Ring chain isomer
Molecules having the same molecular formula possess a ring chain as well as an open structure called Ring chain isomers.
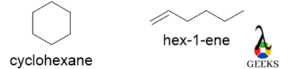
Cyclohexane
Both molecules have the same molecular formula, C6H12 but one has a cyclic structure and an acyclic structure with a double bond. The former has the most stability as the six-member ring is highly stable, so the former has a higher boiling point. The reactivity of both compounds is different because later has greater reactivity due to the presence of a double bond.
Cyclopropane
The compounds have the same molecular formula C3H6, but one is cyclic and the other is long-chain along with a double bond.
We know a three-member structure contains more angle strain (Brett’s rule) so the cyclopropane has lower stability than Prop-1-ene. So, the boiling point of the latter is more than the former. Prop-1-ene has a terminal double bond so the epoxidation occurs easily.

Cyclobutene
For the above molecules, the molecular formula is the same C4H6. But the former is an acyclic structure and an alkyne and the latter is a cyclic alkene. Again, we know a four-member ring has angle strain, so the former is more stable than the latter.
The former has a terminal triple bond, so the reactivity of the former is very high and the boiling point of the former is also very high.
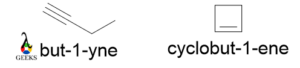
Functional group isomer
Isomers having the same molecular formula but different of the functional group present in it is called functional group isomer. They have different chemical and physical properties.
Functional group isomerism is shown in mainly the alcohol group and ether group. It is a structural isomers examples.
Ether/Alcohol
The molecular formula of these two compounds is the same, C2H6O but in 1st molecule is a primary alcohol group and 2nd compound is ether linkage. The former undergoes nucleophilic substitution reaction only but the latter undergoes nucleophilic substitution as well as elimination reaction. So, the mode of reaction in the two molecules is different. The former has a higher boiling point as there is an ethyl group present as well as it can form H-bond.
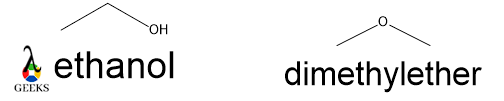
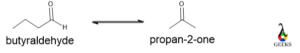
Aldehyde/ketone
Both the molecule has the same molecular formula which is C3H6O but one has aldehyde functionality and the other is a ketone.
Due to steric effect and electronic factor aldehyde is more reactive than ketone center. Aldehyde is easily oxidized to form carboxylic acid but ketone can’t be oxidized to break the carbon chain. Due to long-chain aldehyde having a higher boiling point.
Acid/Ester
Both the molecule have the same molecular formula C3H6O2 but 1st one is acid and 2nd one is an ester. The boiling point of acid is higher than ester due to H-bond. The reactivity of both species is different.
Acid forms due to oxidation of alcohol group and ester are formed via oxidation of ketone group. It is a structural isomers examples.

Tautomerism
Tautomerism is a phenomenon in which an interconversion of H atom and double-bonded atom.
Tautomerism occurs in presence of a catalyst, maybe in presence of acid or base catalyst.
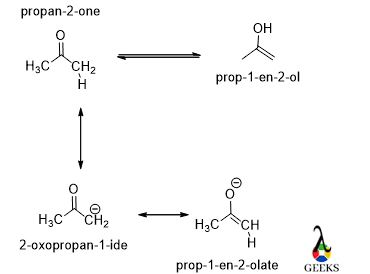
Keto-Enol
Both the molecules have the same molecular formula but the left one has ketone functionality and the right one has alcohol functionality along with the double bond.
The carbon-oxygen double bond is stronger than carbon-carbon, due to this reason keto form is always more stable than the enol form.
So, the keto form is less reactive and the boiling point is higher than the enol form. It is a structural isomers examples.
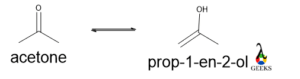
Dicarbonyl
Both the molecule having same molecular formula but the former is dicarbonyl and the latter is keto group along with double bond and -OH group.
So, the physical properties of both compounds are different. For the same reason, keto form is more stable than enol form. Due to the intermolecular H bond, the stability of enol form is higher than the keto form.
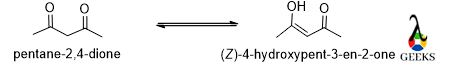
Enolate ion
For enolate ion, keto form is more stable cause the negative charge can undergo resonance with the double bond.
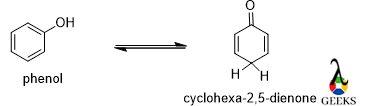
Phenol
In the case of phenol, the enol form is the most stable isomer because it is aromatic in nature but its keto form lost its aromaticity.
- 11 Hydrophobic Examples: Facts You Should Know
- Hypotonic Solution: Definition,Examples,Principles,Effects.
- Exergonic vs Endergonic: Detailed Explanations And Insights
- Oganesson Chemical Properties (25 Facts You Should Know)
- 11 Constitutional Isomers Examples: With Detailed Facts
- Discover The 15 Incredible Facts On HNO3 + BeO Reaction

Hi……I am Biswarup Chandra Dey, I have completed my Master’s in Chemistry from the Central University of Punjab. My area of specialization is Inorganic Chemistry. Chemistry is not all about reading line by line and memorizing, it is a concept to understand in an easy way and here I am sharing with you the concept about chemistry which I learn because knowledge is worth to share it.