A reaction that has a natural urge to take place by itself under a given set of conditions (once it has been imitated if necessary) and moves from a non-equilibrium state to an equilibrium state is called a spontaneous reaction.
- The reaction of Zinc with copper sulfate
- Addition of sodium metal in water
- Melting of ice
- Vaporization of water
- Splitting of a radioactive atom
- The reaction of Barium Hydroxide octahydrate with dry ammonium chloride
- The combustion reaction of coal
- The reaction of Vinegar with Baking Soda
- Rust formation
- Conversion of diamond to graphite
- Expansion of gas into the low-pressure region
- Conduction of heat to the colder object
- Neutralization of strong acid by a strong base
- Diffusion of solute from concentrated to dilute solution
- Cobalt (II) chloride with thionyl chloride
- Vinegar with acid
- Formation of carbon dioxide
- The reaction of acetic acid and ethanol
The reaction of Zinc with copper sulfate
Zinc which is more reactive than copper, undergoes a spontaneous exothermic reaction in which copper is replaced by Zinc and forms Zinc Sulphate.
Zn + CuSO4 –> ZnSO4 + Cu
According to the reactivity, series Zinc is more reactive than copper, so when Zinc is added to copper sulfate solution, copper is replaced by zinc and forms ZnSo4. The blue-colored solution becomes colorless.
Gibbs’s energy and spontaneity
If ∆G (a thermodynamic potential used to calculate the maximum work performed by a closed system) is negative, than the reaction will be spontaneous
It is because the reaction proceeds in the direction in which useful work is performed by the system, causing a decrease in Gibbs energy.
The Gibbs energy change for a process is given by equation
∆G = ∆H – T ∆S
It is obvious that the spontaneity of a process depends upon ∆H, ∆S and T of this process
Some points to note down;
1)If ∆H is negative and ∆S is positive then ∆G will be negative and and the reaction would be definitely spontaneous
2)If both ∆H and ∆S are negative then ∆G would be negative and the process would be spontaneous only at temperatures sufficiently low such that ∆H > T ∆S numerically
3)If both ∆H and ∆S are positive thing would be negative and process would be spontaneous only at temperatures sufficiently high such that ∆H < T ∆S numerically
4) If ∆H is positive and ∆S is negative then ∆G will be positive and the reaction would be definitely non-spontaneous at all temperatures
∆G vale for the above reaction is about -212 at 298K which is negative and hence is a spontaneous reaction.
Addition of sodium metal in water
Sodium metal when added into the water reacts spontaneously with water undergoing exothermic reaction and produce large amount of heat.
Sodium belongs to the group 1 elements in the periodic table and has 1 valence electrons so they are ready to lose this electron to gain noble gas electronic configuration which is highly stable.
So, when sodium is added into the water they readily react and give NaOH and H2 gas with large amount of heat.
2Na(s) + 2H2O –> 2NaOH (Aq) + H2 (gas)
Melting of ice
Melting of ice to water is a spontaneous process in which ice absorbs heats from the surrounding and melts undergoing endothermic spontaneous reaction.
According to second law of thermodynamic increase in entropy of a reaction remains constant for reversible reactions whereas it increases for an irreversible reaction (spontaneous reaction)
Melting of ice is also a spontaneous reaction example.
H2O (ice) –> H2O (Liquid)
The tendency of the ice to melt to liquid state is because the disorder in a liquid is more than in a solid and hence entropy of liquid will be more compared to ice thus undergoes spontaneous reaction.
Vaporization of water
Vaporisation of water to gas increases randomness because and thus entropy and makes the reaction spontaneous.
H2O (liquid) –> H2O (gas)
Vaporisation of water to ice is a endothermic spontaneous reaction in which water absorbs heat from the environment and due to this increase in temperature will vaporise the water to gas increase the disorder in molecules of a gas and also the entropy.
Splitting of a radioactive atom
Spontaneous fission is a spontaneous reaction example in which a unstable nuclei split to fragments with a release of large amount energy.
Spontaneous fission is type of radioactive decay in which an isotope with unstable nuclei split and convert to more stable nuclei by emitting radiation. The rate of the decay of the nuclei varies for different isotopes. It is a good spontaneous reaction example.
cf252 –> Xe140 +Ru108 + 4n + energy
Reaction of Barium Hydroxide octahydrate with dry ammonium chloride
Reaction of Barium Hydroxide octahydrate with dry ammonium chloride is a spontaneous reaction with negative ∆G value.
Ba(OH)2 .8H2O(S) + 2NH4Cl(S) –> 2BaCl2.2H2O (aq) + 8H2O(l) + 2NH3(g)
∆H = 63.5 KJ/ mol
T = 298K
∆S =0.368J/K mol
∆G =∆H -T ∆S
= 63.5 -298 (.368) = -46.1 KJ
Gibbs energy is negative making reaction spontaneous.
The Combustion reaction of coal
Combustion reaction of coal is an spontaneous reaction example and is and is the reason for coal fires in uncontrolled environments.
Coal + O2 –> CO + CO2 +H2O + Heat
Coal has the tendency to self-heat due to its auto oxidation of its compounds making spontaneous combustion the most common cause for uncontrolled burning. There are many coal fires occurring around the world which have been burning for thousands of years
The Reaction of Vinegar with Baking Soda
Reaction of baking soda (NaHCO3) with vinegar (CH3COOH) is an exothermic spontaneous reaction with enthalpy of product formation is lower than that of the enthalpy of reactant
NaHCO3 +CH3COOH –> CO2 + H2O + CH3COONa
Rust formation
Rust formation is a spontaneous exothermic reaction in which the rusting of iron to oxygen takes place in the presence of moist
When an iron or its alloy is exposed to oxygen in presence of moist undergoes a redox reaction between oxygen and iron.
Eg; Corrosion of iron. In this reaction a red layer of oxide iron is formed
Reaction
Fe(s) + 2H+ +1/2 O2 –>Fe2+ + H2O
∆G value for the above reaction is found to be negative and hence is a spontaneous reaction
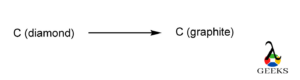
Conversion of diamond to graphite
Conversion of diamond to graphite is a spontaneous process as graphite is more stable allotrope of carbon than diamond
Spontaneous reaction shows the tendency to acquire a state of minimum energy
spontaneous reaction example
Diamond is stable allotrope at very high pressure and in normal conditions the kinetic energy of the particles of diamond is slow to be virtually non-existent.
But they are thermodynamically unstable so they convert to more stable graphite having low energy compared to diamond but due to low kinetic energy reaction is very slow
The process does not require a chemical reaction with an external factor applied.
Expansion of gas into low pressure region
A gas expands spontaneously into a region of low pressure. The reverse process never occurs in its own.
Expansion og gas into low pressure region is a good spontaneous reaction example Flow of gas from high to low pressure region is a natural process and it continues to expands until a state of uniform pressure is achieved.
Conduction of heat to the colder object
Heat is conducted spontaneously along a object from hotter to colder end until the temperature is uniform along the object
If a metal bar is hot at one end and cold at other end heat is conducted from hotter end to colder end and heat flow in reverse direction will not occur.so, a cold object in contact with hot object never gets colder it gets hotter.
Heat energy functions on this principle and convert heat or thermal energy to mechanical energy.
Neutralisation of strong acid by strong base
When strong acid is neutralised by strong base its enthalpy of neutralisation always remain a constant and does not depend on what acid or base is used.
The enthalpy of neutralisation value will be always -57.32KJ/mol. Neutralization of strong acid by strong base is a spontaneous reaction example
Consider the neutralisation of NaOH (strong base) by HCl (strong acid). They are completely ionised in solution state and hence entropy of the reaction will be high.
H+Cl– + Na+OH– –> Na+Cl– +H2O
The ∆G value of the above reaction is negative and hence is a spontaneous reaction.
Diffusion of solute from concentrated to dilute solution
The spontaneous diffusion of a solute from more concentrated solution to a less concentrated solution in contact with it and continues until a state of uniform concentration is attained.
When a concentrated solution of solute is bought into contact with a dilute solution of it, there is a net diffusion of the solute from the former to the latter and not in the other direction.
Cobalt (II) chloride with thionyl chloride
Reaction of Cobalt (II) chloride with thionyl chloride is a spontaneous reaction example as the entropy of the reaction is very high.
COCl2.6H2O(S) + 6SOCl2(l) –> COCl2(s) + 12HCl(g) + 6SO2(g)
In the product formed 12 moles of HCl gas and 6 moles of SO2 gas is formed and because of the entropy which is a measure of disorder will increase and make the reaction feasible i.e. spontaneous.
Vinegar with acid
Reaction of vinegar with acid is a spontaneous reaction as ∆G value for it is negative.
NaHCO3(S) + HCL(aq) –> NaCl(aq) + H2O(l) + CO2
the products formed are in liquid and gaseous state so disorder will be more compared to reactant and hence entropy will also increase making Gibbs energy negative.
Formation of carbon dioxide
Formation of carbon dioxide from carbon monoxide and oxygen is a spontaneous exothermic reaction at standard condition.
CO(g) + 1/2O2(g) –> CO2(g)
The ∆S = ∆S Product – ∆S reactant
=213.65- [197.65 + ½ (205.0)]
-86.55J/K mol
∆G = ∆H – T ∆S
= 282800 J/mol – 298 k (-86.5 J/K mol)
=-257023 J/mol
∆G is negative
The Reaction of acetic acid with ethanol
Reaction of acetic acid with an ethanol is a spontaneous reaction but its enthalpy of reaction is zero.
Acetic acid and ethanol combine together and release water molecule forming ethyl acetate.
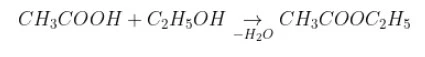
Conclusion –
From the above examples we can conclude that spontaneous reaction is a natural process and cannot be reversed by its own and a reaction can be spontaneous only when entropy is higher than enthalpy of the reaction and ∆G should be negative.
Hi..I am Lina Karankal, I have completed my Master’s in Chemistry. I always like to explore new areas in the field of Chemistry.
Apart from this, I like to read, travel and listen to Music.