Sodium sulfide is a hygroscopic ionic solid with the formula of Na2S. It is corrosive and has a rotten egg-like smell. Let us discuss various uses of Sodium sulfide (Na2S).
Sodium sulfide is used in a variety of fields, including
- Textile Industry
- Leather Industry
- Paper and Pulp Industry
- Photographic Industry
- Rubber Industry
- Cosmetic Industry
- Application of dyes
- Flotation of Ores
- Regeneration of Silver
- Thermoplastic Polymer Preparation
- Oil Refineries
Na2S is readily available in the hydrated form, Na2S. 9H2O. Both anhydrous and hydrous forms are colorless. In this article, we will discuss various uses of sodium sulfide in detail.
Textile Industry
Na2S is used to bleach, dechlorinate, and desulfurize cotton clothes in the textile industry.
Leather Industry
Na2S is used as a dehairing agent in the pre-tanning stage of the leather manufacturing process.
Paper and Pulp Industry
- Na2S is employed in the paper and pulp industries during the Kraft process.
- In this procedure, wood chips are treated with Na2S and a hot solution of sodium hydroxide (NaOH) which dissolve the bonds connecting lignin, hemicellulose, and cellulose in wood.
- The presence of slimes and precipitates in the pulp increases the need for more sodium sulfide to remove them.
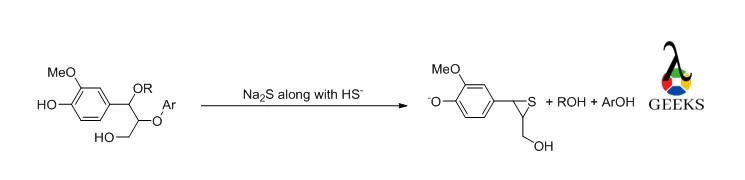
Photographic Industry
Na2S is used to stop the developing solution from oxidizing in the photography industry.
Rubber Industry
The rubber industry uses yellow Na2S flakes to produce high-quality white rubber.
Cosmetic Industry
The cosmetics industry uses Na2S in depilatory creams.
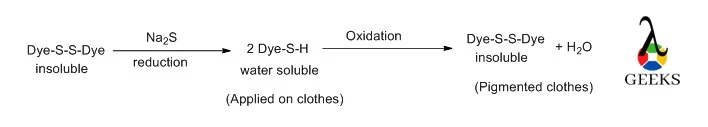
Application of dye
- When applied to clothing, Na2S works as a chemical agent to make insoluble sulfur dyes soluble.
- Sodium sulphide reduces an insoluble pigment, turning it into a liquid substance. At that point, it becomes colorless and is applied to cotton clothes. The pigment and water-insoluble state are then restored after drying and oxidization.
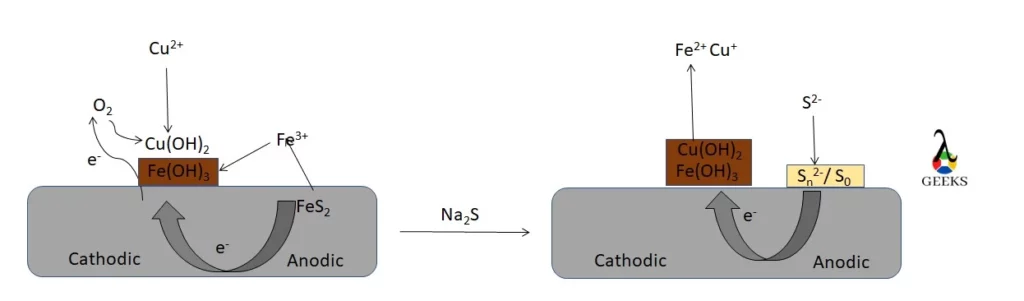
Flotation of ores
- Sphalerite is floated from a copper-zinc ore by activating it with cupric tones in the presence of sodium sulfide ions.
- Chalcopyrite is floated from ore samples wet crushed using iron balls followed by sodium sulfide treatment.
- These findings suggested that the relevant mineral surfaces had been cleaned by Na2S.
Regeneration of Silver
Silver may be recovered from photography trash using Sodium sulphide as re-using the resulting solution becomes challenging in photography.
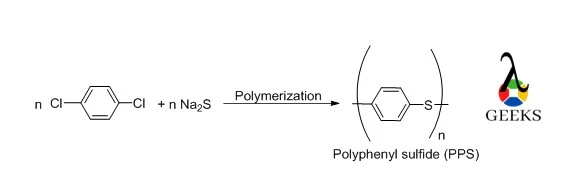
Thermoplastic Polymer Preparation
The interaction of Na2S and 1,4-dichlorobenzene in solvent results in the production of polyphenyl sulfide polymers (PPSs). Filtration, purification, and drying are performed on the polymer produced. PPS can be created in a linear, branching, or cross-linked form.
Oil Refineries
Na2S is used with surfactants to clean up aquifers polluted with nonaqueous phase liquids for oil recovery.
Conclusion
Sodium sulfide (Na2S) is applied in various fields, including water cleaning, leather, pulp, and paper industries, and laboratory reagents for polymerization of its versatile nature.

Hey readers, I am Ishita Ghosh. I have done my Master’s in Chemistry. My area of specialization is Inorganic Chemistry. The true way to comprehend chemistry is to understand it from its grassroots level. My effort is to share every bit of knowledge in chemistry I have so that it helps you for a better grasp on this subject.