Phosphine is a hazardous phosphorous hydride, formed naturally by the anaerobic decay of organic compounds containing phosphorous. It is classed as pnictogen hydride.
Phosphine is flammable (pyrophoric) and it burns with the luminous flame. It is highly toxic compound for the respiratory system and at the concentration of 50ppm it becomes injurious to health. There are several uses of phosphine as it is used as a fumigant, polymerization initiator.
Lavoisier first obtained phosphine in 1783 in the laboratory. Let us see various properties of phosphine like the odor, viscosity, covalent radius, acidic and basic nature in detail.
Phosphine IUPAC name
The IUPAC name of PH3 is phosphane.
Phosphine chemical formula
The chemical formula of phosphine is PH3.
Phosphine CAS number
The CAS number of phosphine is 7803-51-2.
Phosphine ChemSpider ID
The chemSpider ID of phosphine is 22814.
Phosphine chemical classification
- Phosphine contains Phosphorous which belongs to the main group element.
- Phosphine contains phosphorous and is a p-block element and belongs to the Nitrogen family. Hydrogen belongs to the s-block element in the periodic table.
Phosphine molar mass
The molar mass of phosphine is 33.99 g/mol.
Phosphine colour
Phosphine is a colourless gas.
Phosphine viscosity
The viscosity of phosphine is 1.1*10-5. Viscosity is the measure of the resistance of a fluid to deformation.
Phosphine molar density
The molar density of phosphine is 1.379 g/L.
Phosphine melting point
The melting point of phosphine is -132.8oC.
Phosphine boiling point
The boiling point of phosphine is -87.7oC.
Phosphine state at room temperature
Phosphine is gaseous at room temperature and is highly flammable in air.
Phosphine covalent bond
Phosphine has a covalent bond with dipole moment of 0.58 D. This increases with the substitution of methyl groups.
Phosphine covalent radius
The covalent radius of P-H bond is 1.42 Angstrom. It is the measure of the distance between two atoms.
Phosphine electron configurations
Electronic configurations is arrangements of electrons in the shell. Let us see the electronic configuration of Phosphine.
In Phosphine Hydrogen has only one electron so its electronic configuration is 1s1. In phosphorous the electronic configuration is 1s22s22p63s23p6.
Phosphine oxidation state
The oxidation state of phosphorous in Phosphine is -3.
Phosphine acidity/alkaline
Phosphine is amphoteric in nature which means it neither basic nor acidic in nature. It is neutral in nature. Amophoteric species are those which acts as both acids and bases.
Is phosphine odourless?
Phosphine is odourless as pure compound. Commercially it has fishy or garlic smell.
Is phosphine paramagnetic?
Paramagnetic species are those which contains an unpaired electrons. Let us see the Para magnetism of phosphine.
Phosphine is diamagnetic species because of the presence of paired electrons in the orbitals of phosphorous. In the presence of external magnetic field para magnetic species are easily attracted whereas the diamagnetic species are not easily attracted.
Phosphine hydrates
Depending on the conditions Phosphine reacts with the water in different proportions to give mixtures of orthophosphoric acid and phosphorous acid.
Phosphine crystal structure
Phosphine crystal structure is trigonal pyramidal in shape.
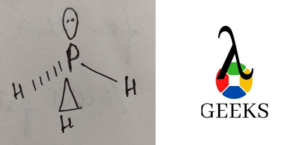
Phosphine polarity and conductivity
- Phosphine is a non-polar compound because of the presence of non-polar P-H bonds.
- Phosphine forms covalent bonds with other compounds hence it is non-conductive.
Phosphine reaction with acid
Phosphine reacts with acids to give phosphonium ions (PH4+). Phosphine has lone pair of electrons. On reaction with an acid which is a H+ donor PH4+ is formed. The reaction with an acid is an irreversible reaction.
PH3 + H+—->PH4+
Phosphine reaction with base
Phosphine reacts with the base to give phosphanide (PH4–) ion and this reaction is reversible in nature.
PH3+ OH–—->PH4–
Phosphine reaction with oxide
Phosphine reacts with the oxygen to give phosphine oxide. It has the chemical formula OPX3. It is tetrahedral in shape. Phosphine oxides are also generated as a by-product in many reaction. For example- In Wittig reactions Phosphine oxides are obtained as a by product.
R3P+1/2 O2 —-> R3PO
Phosphine reaction with metal
Phosphine reacts with metal to give the metal phosphine complex which is also a coordination complex. Metal phosphine complex are also used in homogenous catalysis. For example Wilkinson’s catalyst, Grubbs catalyst.
RhCl3(H2O)3 + 4 PR3 —-> RhCl(PR3)3 + OPPh3 + 2 HCl + 2 H2O
Conclusion
In this article we have discussed about the various properties of the phosphine like the melting point, boiling points, magnetism and various chemical properties like reaction with the metals, reaction with the non-metals and many more.

Hello everyone!! Myself Pragya Anand, I have done my graduation in chemistry (Honours) and Master’s in Organic chemistry. I am a subject matter expert in Chemistry at Chegg. I like exploring all the domains of chemistry and enjoy sharing my knowledge with everyone. Let’s connect through LinkedIn