The PF4 Lewis structure refers to the arrangement of atoms and electrons in a molecule of phosphorus tetrafluoride (PF4). In this structure, phosphorus is the central atom surrounded by four fluorine atoms. The Lewis structure helps us understand the bonding and electron distribution within the molecule. It shows that phosphorus shares its lone pair of electrons with each fluorine atom, resulting in a total of eight valence electrons around the phosphorus atom. This arrangement gives PF4 a trigonal bipyramidal shape. Understanding the PF4 Lewis structure is essential in studying its chemical properties and reactions.
Key Takeaways
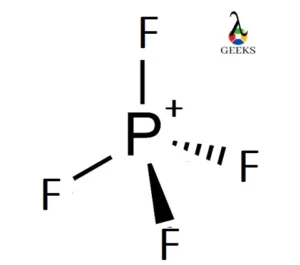
| Lewis Structure | Molecular Shape |
|---|---|
| PF4 | Trigonal Bipyramidal |
Understanding Lewis Structures
Lewis structures are a visual representation of the chemical bonding and electron pairs in a molecule. They provide valuable insights into the arrangement of atoms and the distribution of valence electrons. By understanding Lewis structures, we can determine the molecular geometry, identify covalent bonds, and predict the overall shape of a molecule.
How to Find Lewis Dot Structure
To find the Lewis dot structure of a molecule, we need to follow a few steps. First, we determine the total number of valence electrons in the molecule. Valence electrons are the electrons in the outermost shell of an atom and are crucial for chemical bonding. The number of valence electrons can be determined by referring to the periodic table.
Next, we distribute the valence electrons around the atoms in the molecule, starting with the central atom. The central atom is usually the least electronegative element in the compound. We place one electron pair (represented by a dot) around each atom until all the valence electrons are used.
Determining Valence Shell Electrons
To determine the valence shell electrons, we look at the group number of the element in the periodic table. For example, elements in Group 1 have one valence electron, while elements in Group 2 have two valence electrons. Elements in Groups 13 to 18 have valence electrons equal to their group number minus ten. This method allows us to quickly determine the number of valence electrons for most elements.
Finding Bonding Electrons
Bonding electrons are the electrons involved in the formation of covalent bonds between atoms. In a Lewis structure, bonding electrons are represented by lines or dashes between the atoms. To find the number of bonding electrons in a molecule, we subtract the number of nonbonding electrons from the total number of valence electrons.
Finding Nonbonding Electrons
Nonbonding electrons, also known as lone pairs, are the electrons that are not involved in bonding and are localized on a single atom. These electrons are represented by dots in a Lewis structure. To find the number of nonbonding electrons, we subtract the number of bonding electrons from the total number of valence electrons.
By following these steps, we can construct the Lewis dot structure for various molecules. Let’s take an example of phosphorus tetrafluoride (PF4) molecule. Phosphorus is the central atom, and it has five valence electrons. Each fluorine atom contributes one valence electron. Therefore, the total number of valence electrons in PF4 is 5 + 4 = 9.
To distribute the electrons, we place one electron pair (two electrons) between phosphorus and each fluorine atom, resulting in four bonding electrons. The remaining five electrons are placed as lone pairs on the phosphorus atom. The Lewis dot structure of PF4 is as follows:
F
|
F-P-F
|
F
Understanding Lewis structures and their components, such as valence electrons, bonding electrons, and nonbonding electrons, allows us to visualize the electron configuration and chemical structure of different molecules. These structures are essential for studying molecular models, predicting molecular shape using the VSEPR theory, understanding atomic orbitals’ hybridization, and determining whether a molecule is polar or nonpolar based on its bond angles.
In more complex molecules, resonance structures may be required to represent the delocalization of electrons. These structures show different arrangements of atoms while maintaining the same overall connectivity. They are denoted by double-headed arrows between the different resonance forms.
Lewis structures provide a foundation for understanding the chemical bonding and properties of various compounds. By mastering the art of constructing Lewis structures, we can gain valuable insights into the molecular world and explore the fascinating realm of chemical compounds.
Detailed Analysis of PF4- Lewis Structure
Description of the Lewis Structure of PF4- Ion
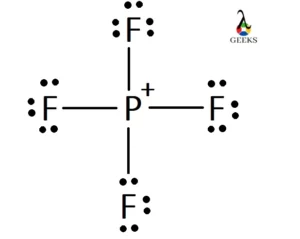
The Lewis structure of PF4- represents the chemical bonding and arrangement of atoms and electrons in the phosphorus tetrafluoride ion. In order to understand the Lewis structure of PF4-, it is important to consider the concept of valence electrons and the octet rule.
Phosphorus tetrafluoride (PF4) is a chemical compound composed of one phosphorus atom (P) and four fluorine atoms (F). The Lewis dot diagram is a visual representation of the valence electrons in an atom or molecule. In the case of PF4-, the Lewis dot diagram shows that phosphorus contributes five valence electrons, while each fluorine atom contributes seven valence electrons.
To determine the Lewis structure of PF4-, we need to count the total number of valence electrons. Phosphorus contributes five valence electrons, and each fluorine atom contributes seven valence electrons, resulting in a total of 32 valence electrons for PF4-.
Next, we arrange the atoms in the structure. Phosphorus is placed in the center, and the four fluorine atoms are positioned around it. Each fluorine atom forms a covalent bond with the phosphorus atom, sharing one pair of electrons. This results in a total of four covalent bonds in the PF4- ion.
Explanation of the Arrangement of Atoms and Electrons in PF4-
The arrangement of atoms and electrons in PF4- can be explained using the VSEPR theory (Valence Shell Electron Pair Repulsion theory) and the concept of hybridization. According to the VSEPR theory, electron pairs in the valence shell of an atom repel each other, causing them to arrange themselves as far apart as possible to minimize repulsion.
In the case of PF4-, the central phosphorus atom undergoes sp3 hybridization, which means that one s orbital and three p orbitals combine to form four sp3 hybrid orbitals. These hybrid orbitals then overlap with the p orbitals of the fluorine atoms, resulting in the formation of four sigma bonds.
The molecular geometry of PF4- is tetrahedral, with the phosphorus atom at the center and the four fluorine atoms positioned at the corners of a tetrahedron. The bond angles between the phosphorus and fluorine atoms are approximately 109.5 degrees, as predicted by the VSEPR theory.
Importance of Counting Valence Electrons and Following Octet Rule in PF4-
Counting valence electrons and following the octet rule are crucial steps in determining the Lewis structure of PF4- and understanding its chemical properties. Valence electrons are the outermost electrons in an atom, and they play a significant role in chemical bonding.
By counting the valence electrons in PF4-, we can determine the total number of electrons available for bonding. This information helps us determine the number of covalent bonds that can be formed and the arrangement of atoms in the molecule.
Following the octet rule ensures that atoms achieve a stable electron configuration by having a full outer shell of eight electrons (except for hydrogen and helium, which follow the duet rule). In the case of PF4-, the phosphorus atom achieves an octet by forming four covalent bonds with the fluorine atoms, while each fluorine atom also achieves an octet.
Understanding the Lewis structure, molecular geometry, and electron configuration of PF4- allows us to predict its chemical behavior and interactions with other compounds. It provides insights into the polarity of the molecule, bond angles, and resonance structures, which are essential for understanding its properties and reactivity.
Hybridization in Lewis Structures
How to Find Hybridization from Lewis Structure
In chemical bonding, the arrangement of electron pairs around an atom plays a crucial role in determining the molecular geometry and the type of covalent bonds formed. Lewis structures, also known as Lewis dot diagrams, provide a visual representation of the valence electrons and the bonding patterns in a molecule. However, they do not provide information about the three-dimensional shape of the molecule. This is where hybridization comes into play.
Hybridization is a concept that helps us understand the molecular geometry of a compound by combining the ideas of atomic orbitals and the electron configuration. It involves the mixing of atomic orbitals to form new hybrid orbitals, which then participate in the formation of chemical bonds. These hybrid orbitals have different shapes and orientations, allowing us to predict the bond angles and molecular shape.
To determine the hybridization of an atom in a Lewis structure, we can follow a simple step-by-step process:
- Count the number of electron groups (bonding pairs and lone pairs) around the central atom.
- Determine the steric number by adding the number of bonding pairs and lone pairs.
- Use the steric number to identify the hybridization of the central atom.
The steric number corresponds to the number of hybrid orbitals formed during hybridization. Here are some common steric numbers and their corresponding hybridizations:
- Steric number 2: sp hybridization (linear geometry)
- Steric number 3: sp2 hybridization (trigonal planar geometry)
- Steric number 4: sp3 hybridization (tetrahedral geometry)
- Steric number 5: sp3d hybridization (trigonal bipyramidal geometry)
- Steric number 6: sp3d2 hybridization (octahedral geometry)
Explanation of Hybridization and its Role in Determining the Shape of a Molecule
Hybridization is essential in determining the shape of a molecule because it influences the arrangement of atoms and the bond angles. The VSEPR (Valence Shell Electron Pair Repulsion) theory helps us predict the molecular shape based on the number of electron groups around the central atom. By knowing the hybridization, we can determine the number and arrangement of these electron groups.
For example, let’s consider the molecule phosphorus tetrafluoride (PF4). The Lewis structure of PF4 shows that there are four bonding pairs around the central phosphorus atom, resulting in a steric number of 4. According to our earlier discussion, a steric number of 4 corresponds to sp3 hybridization.
In PF4, the sp3 hybrid orbitals of phosphorus overlap with the p orbitals of the fluorine atoms, forming four sigma bonds. The resulting molecular shape is tetrahedral, with bond angles of approximately 109.5 degrees. This information allows us to understand the chemical structure and molecular models of PF4.
Determination of Hybridization in PF4- Based on its Lewis Structure
Now, let’s consider the negatively charged ion PF4-. The Lewis structure of PF4- shows that there is an additional lone pair of electrons on the central phosphorus atom, making the steric number 5. A steric number of 5 corresponds to sp3d hybridization.
In PF4-, the sp3d hybrid orbitals of phosphorus overlap with the p orbitals of the fluorine atoms, forming four sigma bonds, similar to PF4. However, the additional lone pair affects the molecular shape. The lone pair occupies more space, causing the fluorine atoms to be pushed closer together. As a result, the bond angles in PF4- are slightly less than 109.5 degrees.
Comparison of PF4- Lewis Structure with Other Molecules
PN Lewis Structure and Comparison with PF4- Lewis Structure
When it comes to chemical bonding and the arrangement of electron pairs, the Lewis structure is a valuable tool. It allows us to visualize the distribution of valence electrons in a molecule and understand its molecular geometry. In this comparison, we will explore the Lewis structure of PF4- and compare it with the Lewis structure of PN.
The PF4- molecule, also known as phosphorus tetrafluoride, consists of one phosphorus atom bonded to four fluorine atoms. To determine its Lewis structure, we start by considering the electron configuration of phosphorus, which has five valence electrons. Each fluorine atom contributes one valence electron, resulting in a total of nine valence electrons for PF4-. By following the octet rule, we can distribute these electrons around the central phosphorus atom, resulting in a structure where each fluorine atom is bonded to phosphorus.
On the other hand, PN, or phosphorus nitride, is a chemical compound composed of one phosphorus atom bonded to one nitrogen atom. The Lewis structure of PN is determined by considering the electron configuration of both phosphorus and nitrogen. Phosphorus has five valence electrons, while nitrogen has five. By sharing one electron pair, the phosphorus and nitrogen atoms form a covalent bond, resulting in a structure where both atoms have achieved an octet.
To compare the PF4- Lewis structure with the PN Lewis structure, we can examine their molecular models and molecular shapes. According to the VSEPR theory, the electron pairs around the central atom in PF4- repel each other, resulting in a tetrahedral molecular shape. The bond angles between the phosphorus and fluorine atoms are approximately 109.5 degrees. On the other hand, the PN molecule has a linear molecular shape due to the presence of only one bond between phosphorus and nitrogen.
Another aspect to consider is the presence of resonance structures and hybridization. In PF4-, there are no resonance structures since all the fluorine atoms are equivalent. However, in PN, resonance structures can be formed due to the possibility of electron delocalization between the phosphorus and nitrogen atoms. This delocalization leads to the hybridization of atomic orbitals, resulting in a more stable structure.
In terms of polarity, PF4- is a polar molecule due to the presence of fluorine atoms, which are more electronegative than phosphorus. This uneven distribution of charge creates a dipole moment. On the other hand, PN is a nonpolar molecule since the electronegativity difference between phosphorus and nitrogen is relatively small.
To summarize, the comparison of PF4- Lewis structure with PN Lewis structure reveals differences in molecular geometry, resonance structures, hybridization, and polarity. While PF4- adopts a tetrahedral shape with no resonance structures, PN has a linear shape with the possibility of resonance. Understanding the Lewis structures and molecular properties of these molecules provides valuable insights into their chemical behavior.
F3- Lewis Structure and Comparison with PF4- Lewis Structure
In addition to comparing the PF4- Lewis structure with PN, let’s now explore the Lewis structure of F3- and compare it with the Lewis structure of PF4-.
The F3- molecule consists of one central fluorine atom bonded to three additional fluorine atoms. To determine its Lewis structure, we consider the electron configuration of fluorine, which has seven valence electrons. By following the octet rule, we distribute these electrons around the central fluorine atom, resulting in a structure where each fluorine atom is bonded to the central fluorine atom.
Comparing the F3- Lewis structure with the PF4- Lewis structure, we can observe similarities in their molecular geometry. Both molecules adopt a tetrahedral shape due to the repulsion between the electron pairs around the central atom. However, the bond angles in F3- are slightly smaller than in PF4-, approximately 109 degrees.
When it comes to resonance structures and hybridization, F3- does not exhibit any resonance structures since all the fluorine atoms are equivalent. Additionally, there is no hybridization of atomic orbitals in F3-. On the other hand, PF4- does not have resonance structures but does exhibit hybridization due to the presence of phosphorus.
In terms of polarity, F3- is a polar molecule due to the electronegativity difference between fluorine and the central fluorine atom. This uneven distribution of charge creates a dipole moment. Similarly, PF4- is also a polar molecule due to the electronegativity difference between phosphorus and fluorine.
To summarize, the comparison of F3- Lewis structure with PF4- Lewis structure reveals similarities in molecular geometry but differences in resonance structures, hybridization, and polarity. Both molecules adopt a tetrahedral shape, but only PF4- exhibits hybridization and the possibility of resonance. Understanding the Lewis structures and molecular properties of these molecules helps us comprehend their chemical behavior.
Frequently Asked Questions
How do you find the Lewis dot structure?
Finding the Lewis dot structure involves understanding the concept of chemical bonding and electron pairs. The Lewis dot structure, also known as the Lewis structure or Lewis dot diagram, is a representation of the valence electrons in an atom or molecule. To find the Lewis dot structure, you need to follow these steps:
- Determine the total number of valence electrons for the atom or molecule.
- Place the least electronegative atom in the center and connect it to the surrounding atoms using single bonds.
- Distribute the remaining electrons around the atoms, giving each atom an octet (except hydrogen, which only needs 2 electrons).
- If there are still remaining electrons, place them on the central atom as lone pairs.
- Check if all atoms have an octet or a duet (for hydrogen). If not, you may need to form double or triple bonds to achieve stability.
What is the Lewis structure of PF4-?
The Lewis structure of PF4- (phosphorus tetrafluoride ion) can be determined by following the steps mentioned earlier. Phosphorus (P) is the central atom, and it is bonded to four fluorine (F) atoms. Phosphorus has 5 valence electrons, while each fluorine atom has 7 valence electrons. Therefore, the total number of valence electrons in PF4- is 5 + (4 × 7) + 1 (for the negative charge) = 32.
To draw the Lewis structure of PF4-, place the phosphorus atom in the center and connect it to the four fluorine atoms using single bonds. Distribute the remaining electrons around the atoms, giving each atom an octet. In this case, the phosphorus atom will have an expanded octet, meaning it will have more than 8 electrons. The Lewis structure of PF4- will have 32 valence electrons arranged accordingly.
How to solve Lewis dot structures?
Solving Lewis dot structures involves understanding the concept of chemical bonding, electron configuration, and the octet rule. Here are the steps to solve Lewis dot structures:
- Determine the total number of valence electrons for the atom or molecule.
- Identify the central atom, usually the least electronegative atom, and connect it to the surrounding atoms using single bonds.
- Distribute the remaining electrons around the atoms, giving each atom an octet (except hydrogen, which only needs 2 electrons).
- If there are still remaining electrons, place them on the central atom as lone pairs.
- Check if all atoms have an octet or a duet (for hydrogen). If not, you may need to form double or triple bonds to achieve stability.
- Consider resonance structures if applicable, where electrons can be delocalized to different positions.
- Determine the formal charges on each atom to ensure the overall charge of the molecule is balanced.
By following these steps, you can solve Lewis dot structures and understand the chemical structure and bonding in different molecules.
How to find hybridization from Lewis structure?
To find the hybridization of an atom in a molecule using the Lewis structure, you need to consider the number of electron groups (bonded and lone pairs) around the atom. Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals, which influences the molecular geometry and bond angles. Here’s how you can find hybridization from a Lewis structure:
- Count the number of electron groups (bonded and lone pairs) around the atom of interest.
- Determine the steric number, which is the sum of the number of bonded atoms and lone pairs.
- Use the steric number to determine the hybridization of the atom:
- Steric number 2: sp hybridization
- Steric number 3: sp2 hybridization
- Steric number 4: sp3 hybridization
- Steric number 5: sp3d hybridization
- Steric number 6: sp3d2 hybridization
The hybridization of an atom affects its shape and bond angles, which in turn influences the overall molecular geometry of the molecule.
What is the molecular geometry of PF4?
The molecular geometry of PF4 (phosphorus tetrafluoride) can be determined using the Valence Shell Electron Pair Repulsion (VSEPR) theory. In PF4, the central phosphorus atom is bonded to four fluorine atoms. Since there are no lone pairs on the central atom, the molecular geometry of PF4 is tetrahedral.
In a tetrahedral molecular geometry, the bond angles between the bonded atoms are approximately 109.5 degrees. This arrangement ensures that the electron pairs are as far apart as possible, minimizing repulsion and maximizing stability.
How to work out Lewis structure?
Working out the Lewis structure of a molecule involves following the steps mentioned earlier. To summarize, here’s how you can work out the Lewis structure:
- Determine the total number of valence electrons for the atom or molecule.
- Identify the central atom, usually the least electronegative atom, and connect it to the surrounding atoms using single bonds.
- Distribute the remaining electrons around the atoms, giving each atom an octet (except hydrogen, which only needs 2 electrons).
- If there are still remaining electrons, place them on the central atom as lone pairs.
- Check if all atoms have an octet or a duet (for hydrogen). If not, you may need to form double or triple bonds to achieve stability.
- Consider resonance structures if applicable, where electrons can be delocalized to different positions.
- Determine the formal charges on each atom to ensure the overall charge of the molecule is balanced.
By following these steps, you can work out the Lewis structure of a molecule and understand its chemical bonding and structure.
What is the Lewis structure of PF4-1?
There is a typo in the question. It should be PF4- instead of PF4-1. The Lewis structure of PF4- (phosphorus tetrafluoride ion) has been discussed earlier. It consists of a central phosphorus atom bonded to four fluorine atoms. The Lewis structure of PF4- will have 32 valence electrons arranged accordingly. The negative charge on the ion indicates the addition of one extra electron to the structure, resulting in a total of 32 valence electrons.
The Lewis structure of PF4- can be determined by following the steps mentioned earlier, considering the additional electron and adjusting the formal charges accordingly.
Frequently Asked Questions
1. What is the Lewis structure of PF4- and what does its molecular geometry look like?
The Lewis structure for PF4- involves a central phosphorus atom surrounded by four fluorine atoms, each sharing a single covalent bond with the phosphorus. This results in a total of 8 valence electrons around the phosphorus atom, satisfying the octet rule. The molecular geometry of PF4- is tetrahedral, according to the VSEPR theory.
2. How is the PN Lewis structure determined?
The PN Lewis structure is determined by counting the total number of valence electrons for the phosphorus (P) and nitrogen (N) atoms. Phosphorus has 5 valence electrons and nitrogen has 5 as well. These 10 electrons are then arranged to satisfy the octet rule, resulting in a triple bond between P and N with one lone pair on each atom.
3. How can I draw the PF4-1 Lewis structure?
The PF4-1 Lewis structure can be drawn by placing the phosphorus atom in the center and surrounding it with four fluorine atoms. Each fluorine atom shares one electron with phosphorus to form a covalent bond. The extra electron (the “-1” in PF4-1) is placed on the phosphorus atom, resulting in a total of 9 valence electrons around the phosphorus.
4. What is the P4 Lewis structure?
The P4 Lewis structure, also known as the Lewis dot structure for P4 (white phosphorus), involves four phosphorus atoms arranged in a tetrahedral shape. Each phosphorus atom is bonded to the other three, forming a total of six covalent bonds.
5. How do you find the Lewis dot structure of P3-?
The Lewis dot structure of P3- can be found by counting the total number of valence electrons. Each phosphorus atom contributes 5 electrons, and the extra 3 electrons come from the charge of the ion. These 18 electrons are then arranged to satisfy the octet rule for each atom.
6. How can I determine the hybridization from a Lewis structure?
The hybridization of an atom in a molecule can be determined by counting the number of sigma bonds and lone pairs of electrons around the atom. For example, if an atom has 3 sigma bonds and 1 lone pair, it is sp3 hybridized.
7. What is the Lewis dot structure for NL3?
The Lewis dot structure for NL3 involves a nitrogen atom in the center surrounded by three lithium atoms. Each lithium atom shares one electron with nitrogen to form a covalent bond, satisfying the octet rule for nitrogen.
8. How do you draw the PF4 Lewis structure?
The PF4 Lewis structure can be drawn by placing the phosphorus atom in the center and surrounding it with four fluorine atoms. Each fluorine atom shares one electron with phosphorus to form a covalent bond, satisfying the octet rule for phosphorus.
9. What is the Lewis structure for PF4 3-?
The Lewis structure for PF4 3- involves a central phosphorus atom surrounded by four fluorine atoms, each sharing a single covalent bond with the phosphorus. The extra 3 electrons (the “3-” in PF4 3-) are placed on the phosphorus atom, resulting in a total of 11 valence electrons around the phosphorus.
10. How do you solve Lewis dot structures?
To solve Lewis dot structures, start by counting the total number of valence electrons from all atoms in the molecule. Arrange the atoms with the least electronegative atom in the center. Then, draw single bonds between atoms and distribute the remaining electrons to satisfy the octet rule. If there are not enough electrons to satisfy the octet rule for all atoms, try forming double or triple bonds.
Also Read:
- Co lewis structure
- Icl3 lewis structure
- Li2o lewis structure
- Na3po4 lewis structure
- Cr2o3 lewis structure
- Xecl4 lewis structure
- Licl lewis structure
- Ci4 lewis structure
- Pi3 lewis structure
- So3 lewis structure

Hello,
I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202