Oxidising agent example refers to reduced elements, which separate electrons from other elements in Redox reactions. Let us describe the examples.
The Oxidising agent examples are listed below:
- Halogen elements (Cl, Br, I, F)
- Oxygen (O2)
- Hydrogen peroxide (H2O2)
- Potassium permanganate (KMnO4)
- Potassium dichromate (K2Cr2O7)
- Nitric acid (HNO3)
- Carbon dioxide (CO2)
- Concentrated Sulphuric Acid (H2SO4)
- Nitrogen dioxide (NO2)
- Water (H2O)
- Copper (Cu2+)
- Regular Salt (NaCl)
- Acetone (C3H6O)
Halogen elements (Cl, Br, I, F)
Electron affinity refers to an urge of element to have electrons in the empty space of its valence shells. Electronegativity of elements refers to urge of taking electrons in itself. The tendency of halogens to attract the electrons and making halide ions is the key reason that makes them strong oxidising agent.
Oxygen (O2)
Oxygen is the most effective example of oxidising agent. This element is highly valuable in the regular life of people. It works as an oxidiser being reduced while making rust, supporting the human body in breathing and facilitating living beings to do aerobic respiration. It is considered as oxidiser.
Hydrogen peroxide (H2O2)
Hydrogen peroxide is an oxidising agent as it can be reduced readily to convert Carboxylic acid to peroxy acid. However, Hydrogen peroxide is the same kind of oxidiser as oxygen but is more powerful than Oxygen. As this compound holds one more Oxygen, it impose strong oxidation than normal Oxygen molecule.
Potassium permanganate (KMnO4)
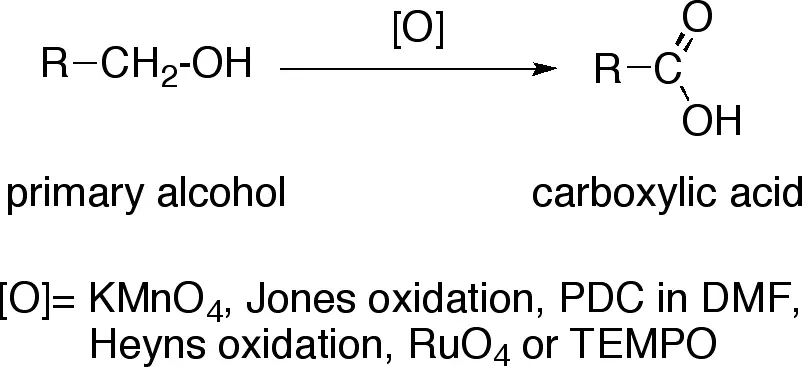
KMnO4 is a reliable example of strong oxidising agent. The oxidation state of the metal (Mn) is +7, which is usually high. This metal drives the tendency of losing electrons easier for the overall compound. The compound mainly get reduced to Carboxylic acid in the chemical reactions.
Potassium dichromate (K2Cr2O7)
Potassium dichromate is an example of strong oxidising agent as it oxidises Hydroxyl ions and many other compounds. Most of the time, the compound use to be reduced in acidic mediums. Generally, it is used in chemical laboratories to oxidise the compounds to give out aldehydes and carboxylic acids.
Nitric acid (HNO3)
The oxidation state of Nitrogen in Nitric acid is +5. This fact notes that the compound is highly capable of taking up electrons. The oxidation state of nitrogen cannot be more than +5. It fluctuates under the range of -3 to +5. It indicates the compound is a suitable electron acceptor than an electron donor.
Carbon dioxide (CO2)
CO2 is the example of Oxidising agent. It is naturally gives out Glucose in present of water that is called Photosynthesis reaction. It happens in chlorophyll contained leaves of green plants. Carbon dioxide readily gains electron and makes the oxidation process active in photosynthesis.
Concentrated Sulphuric Acid (H2SO4)
Sulphuric acid in concentrated form is an example of strong oxidising agent. It is highly reactive but not more than Nitric acid. H2SO4 is a strong Bronsted acid. It tends to react with amphoteric base water and produces H3O+ ions. This is an effective property of oxidising agents.
Nitrogen dioxide (NO2)
Nitrogen oxide is a great common example of oxidising agent. This oxide is noticed to be decomposed in presence of heat. It gives out Nitrogen and oxygen. Nitrogen has oxidation number of +2 to +5. In reaction with water NO2 readily gives out HNO3 which is also found an a strong oxidising agent.
Water (H2O)
Water is also considered as an example of oxidising agent. Though this amphiprotic compound is more reliable in donating electrons, in presence of strong electron donor the compound acts as an oxidising agent. A strong reducing agent can influence the compound to accept electron and form H3O+.
Copper (Cu2+)
Cu2+acts as an oxidising agent in special case of redox reaction. Only one potential redox reaction proofs that Copper is good oxidising agent too. In the form of Copper oxide, the metal involves itself in the reaction of hydrogen gas. When hydrogen gas forms water, Copper act as an oxidising agent in the reaction.
Regular Salt (NaCl)
Regularly used salt as the most essential cooking material is also a good an oxidiser. NaCl is reduced in presence polar solvent such as water. The compound easily gives out base such as NaOH and acid like hydrochloric acid. Here, both the product of reaction between NaCl and Water are reducing agent.

Acetone (C3H6O)
Acetone is an example of organic oxidising agent. This compound get reduced in the reaction between Sodium borohydrate and Acetone. Hydride ion are noticed to act as an effective reducing agent by donating electrons to Acetone, where the second carbon contains two positive charges having urge for electrons.
We can find that all the strong oxidising agents are reactive enough naturally. These occur as the potential active components for chemical reactions and each has strong beneficial role in chemistry to enhance the chemical balances in redox reactions.
- Lewis Structures: Facts You Must Know
- H2SO4 + NaOH: A Powerful Chemical Reaction Unveiled
- Valence Electrons: The Key to Understanding Chemical Reactions
- Sodium Carbonate: Understanding Its Uses, Benefits, and Safety
- Electronegativity Unveiled: Understanding Its Impact on Chemical Bonds

Hi…..I am Sarnali Mukherjee, a graduate from the University of Calcutta. I love to teach and share knowledge on chemistry. I have gradually gained interest in article writing since one year ago. I would love to acquire more knowledge on my subject in the future.
Let’s connect through LinkedIn: