This article discusses NO lewis structure and its hybridization, shape, bond angle, and detailed explanations.
NO is covalent molecule. The central N atom in NO is sp2 hybridized. The molecule is linear-shaped having a bond angle of 1800. There is a partial triple bond character between N and O. In the NO lewis structure we can see the electron distribution as well as lone pairs.
Some facts about Nitric Oxide
The molecular weight of Nitric Oxide is 30.006 g/mol. The density of N2O is 1.3402 g/L. The melting point and boiling point of Nitrous oxide are 109 K and 121 K respectively.
In the laboratory, Nitric Oxide is prepared by the reduction of Nitric acid with Copper.
8 HNO3 + 3 Cu → 3 Cu(NO3)2 + 4 H2O + 2 NO
This is the most common method to synthesize Nitric Oxide.
NO is a strong filed ligand and it can bind with metal strongly having a low oxidation state. It is very toxic and it can bind with iron in hemoglobin and increase toxicity in the human body which results in death.
Method of drawing the Lewis structure for NO
First, we should count the total number of valance electrons to draw the lewis structure of Nitrous Oxide.
In this structure of Nitrous Oxide, we can see that one N and one O atom is present. Now central atom is decided by the least electronegativity. N is less electronegative than O, So N is the central atom here. O is covalently bonded with N and valence electrons are showing in order to complete their octet. To satisfy the octet after putting all the valence electrons we should use a double bond or triple bond accordingly.
NO lewis structure
One lone pair over N atom and two lone pairs over O atom. There is a single electron on the N atom as N contains five electrons in the valence shell, in order to gain stability the free-electron over N forms a bond with O and there will be a partial triple character observed in this molecule.
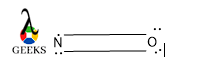
NO valence electron
In Nitric oxide, the N atom is bonded by the O atom. The electronic configuration of N is 1s22s22p3 and the electronic configuration of O is 1s22s22p4. So, taking into consideration the valence shell electron of N is five, among them, two electrons form bond O and two of them remain as a lone pair and one free electron.

So, the total number of valence electrons of N2O is 5 + 6 = 11.
NO lewis structure formal charge
The formal charge is calculated using following formula,
F.C. = Nv – Nl.p. -1/2 Nb.p.
Nv = number of electrons in the valence shell of the free atom
Nl.p = number of electrons in lone pair
Nb.p = number of electrons involved in the bond formation.
From resonance the most contributing structure of Nitric Oxide is
F.C. of N = 5-3-(4/2) = 0
F.C. of O = 6-4-(4/2) = 0
Number of lone pairs in NO lewis structure
The total number of lone pairs is calculated by the sum of an individual atom’s lone pair after bond formation.
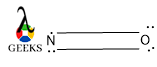
So, it is clear after forming a double bond there are a total of three [(1+2)=3] pairs of lone pairs available. Two of them over O and one of them over N.
Hybridization of NO
No is a diatomic molecule having an odd electron. From the data of bond length between N and O which is equal to 1.15 Å, it was thought that hybridization is sp2 as the bond length is in between double and triple bonds. But we can see n cannot fulfill its octet.
If we consider O complete in its octet then hybridization is sp2 according to the formula,
H = 0.5(V+M-C+A) , where H= hybridization value, M = monovalent atoms, C=cation, A=anion.
But this is not the ultimate solution though there is some controversy about the hybridization of Nitric Oxide.
| Structure | state of hybridization of central atom | Bond angle |
| Linear | sp /sd / pd | 1800 |
| Planner trigonal | sp2 | 1200 |
| Tetrahedral | sd3/ sp3 | 109.50 |
| Trigonal bipyramidal | sp3d/dsp3 | 900 (axial), 1200(equatorial) |
| Octahedral | sp3d2/ d2sp3 | 900 |
| Pentagonal bipyramidal | sp3d3/d3sp3 | 900,720 |
From this table, it is told that as the bond angle of NO is 1800, so it is sp hybridized.
NO bond angle
The bond angle between N and O is 1800. This data is also confirmed by the hybridization of this molecule. The molecule is sp hybridized so the structure of the molecule is linear.
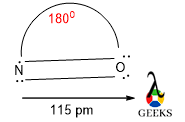
The bond length is 1.15 Å, this value is between the double bond and triple bond. So the geometry is linear and the bond angle will be 1800.
NO octet rule
In Nitrous Oxide N cannot fulfill its octet. As there is an odd electron present in this molecule. The outermost electron for N is five as N is the VA element.
So, two of them electrons make the bond with O ( one is sigma and another is π bond) and two electrons reside as lone pair after that there will be one odd electron remaining. In order to gain stability N can form a triple bond with O by donating that odd electron. This way Nitrous oxide completes its octet.
NO resonance structure
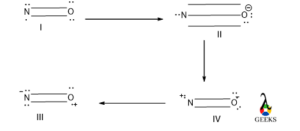
From the above canonical form Structure, II is the most contributing structure as it contains more number of covalent bonds and a negative charge is on the electronegative atom. Structure III is less contributing because unlike charge is over the electronegative atom.
Uses of NO
Nitric Oxide can dilate blood vessels and control high blood pressure.
Frequently asked questions (FAQ)
What are metal nitrosyls?
Metal Nitrosyls are one class of organometallic compounds.
When Nitric Oxide coordinates with Metal then it is called Metal nitrosyls. There Metal nitrosyl can participate in different types of reactions.
What is the correct order of bond strength for No, NO+, NO–?
NO+ > NO > NO–
The Bond order of NO+, NO, N and O– are 3, 2.5, and 2 respectively. The higher the bond order higher will be the bond strength and the lower will be the bond distance.
So, the order of Bond length is just reversed.
Also, please click to know XeO2F2 Lewis Structure and Stearic Acid Structure.
Also Read:
- Ocs lewis structure
- Xecl2 lewis structure
- Ncl4 lewis structure
- Benzoic acid lewis structure
- Caco3 lewis structure
- Pf3 lewis structure
- Cn lewis structure
- Xef2 lewis structure
- Lewis structures
- Ch3nh2 lewis structure

Hi……I am Biswarup Chandra Dey, I have completed my Master’s in Chemistry from the Central University of Punjab. My area of specialization is Inorganic Chemistry. Chemistry is not all about reading line by line and memorizing, it is a concept to understand in an easy way and here I am sharing with you the concept about chemistry which I learn because knowledge is worth to share it.