Monatomic ions are generated specially as electrolytes. “Mono” is a Greek word which here stands for one and “atomic” is the adjective form of atom which is the most tiny part of the matter. Ions are the particles which carry charge over it means number of protons and electrons are not equal.
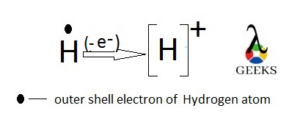
H+ as monatomic ion
Hydrogen is an element of group 1 with atomic symbol “H” which has one electron in the only 1s orbital.
So this element can donate this electron results in the monatomic cation. This is called “H plus” or “Hydrogen plus” ion (H+).
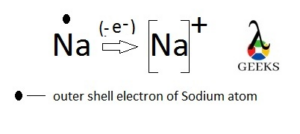
Na+ as monatomic ion
Sodium (Na) is an element of group 1in the third period of the Periodic table which has one electron in the last filled 3s orbital.
It tends to transfer this electron to get the stability like nearest inert gas Neon with electronic configuration 1s2 2s2 2p6. This makes monatomic cation. As the number of proton in the nucleus are more than the electrons moving in various energetic orbits. This ion called “Sodium plus” or Na+.
During electrolysis from table salt this monatomic cation is also generated in the solution. As it carries charge it can move electricity in the solution.
Al3+ as monatomic ion
Aluminum is an element of group number 13 in the third period. 3s and 3p are the last orbitals which have electrons in the atom.
It can remove the three electrons for gaining energetic stability which make a monatomic ion example “Al3+” or “Aluminum 3+”.
K+ as monatomic ion
Potassium is an element in group one of the fourth period. Nearly similar to the nearest inert gas electronic configuration it has one extra electron in 4s orbital.
For energetically stability purpose it remove the electron from the last filled 4s orbital, results in monatomic cation “K+” or “Potassium +”.
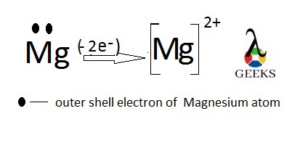
Mg2+ as monatomic ion
Magnesium is in 2nd group of the 3rd period with two electrons in the outer filled 3s orbital.
Removing these electrons it makes monatomic cation “Mg2+” which is most important in Chlorophyll formation.
H- as monatomic ion
As we discuss Hydrogen atom has one electron, so it can also accept another one and make 1s orbital full filled.
This leads in the formation of monatomic anion called “Hydride” ion, which is also a Hydrogen monatomic ion example.
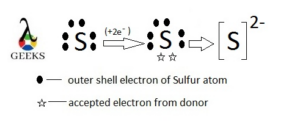
S2- as monatomic ion
Sulfur is in group 16 of the 3rd period with less of two electrons in last filled 3p orbital.
Taking two electrons in 3p orbital, lead to a monatomic anion “Sulfide” formation.
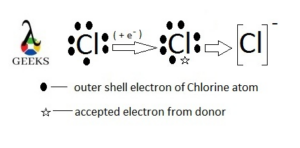
Cl- as monatomic ion
Chlorine belongs to the group 17 of the 3rd period with vacancy of one electron in 3p orbital so that it faces lack of stability.
By accepting one electron it produces monatomic ion example that is “Chloride”. It can be obtain in the electrolysis of table salt.
How monatomic ions form?
Octet rule governs the cause of the formation of ions.
The energy of the orbit of atoms or molecules is most stable when the last occupied orbit fills eight electrons which is also known as Octet rule in atomic structure chemistry. Only group 18 elements in Periodic table have this type of configurational stability.
Except inert gases all atoms in Periodic table have lesser number of electrons in the last filled shell, among which some gain electrons to reach the target or some lose electrons for that. This results in the formation of the single charged atom.
Sometimes molecules especially ionic compounds dissociate and produce simplest elements which have charge over them. Some of the elements belong to the monatomic ion examples. Like NaCl (table salt) dissolves in water and produce two monatomic ions.
Types of monatomic ions
Monatomic ion example contains two categories such as Cation and Anion.
Ions can be classified on the nature of the charge it carries. The nature of the charge which the generating ion occupies depends on the electronic arrangement of the outer most filled orbit of that atom.
Formation of monatomic cation
If the last filled orbital of an atom has less number of electrons than its capacity of the half filled shell then the atom tends to transfer the electron to achieve electronic stability. After the transformation the atom has greater number of protons in nucleus than the electrons results cation.
As cations have greater number of protons so they are positively charged elements. Naturally metals form this cations as they have less than or equal to three loosely bounded electrons by nuclear force in the outer orbit. The charge have to be integer number like (+1), (+2) etc.
In a solution these ions with positive charge are attracted towards cathode, these are called cation.
Formation of monatomic anion
If the last filled orbital of an atom has greater number of electrons than the half filled of that orbital can posses, but not full fill the Octet rule then the atom accepts electrons from donor. This acceptance results in formation of anion.
There is greater number of electrons present in the orbit of an anion than the protons in the nucleus of it so the anions are negatively charged particles. The charge of the anion must be integer in number like (-1), (-2) etc.
In solution these are attracted toward the anode, these are called anion.
How to write monatomic ions?
To write this two things have to be considered, one is the atomic symbol of the ion and then have to predict the charge.
The charge gained by the atom after losing or gaining electrons, is shown at the upper right side of the atomic symbol. Sometime in the monatomic ion examples the oxidation state is written in bracket after the atomic symbol of the monatomic ion in Roman numeral.
As an example we can check Lithium as a monatomic ion. This atomic symbol is “Li”. After losing the 2s orbital electron it produce Li+ monatomic ion.
How to name monatomic ions?
There is a particular naming pattern for both positively and negatively charged monatomic ions which are produced from metals or alkali earth metals and non-metals respectively.
For monatomic cation, the name remains same as the name of the element. The positive charge number is attached with the element name. Monatomic ion of Hydrogen is written as “H+” which is called “Hydrogen +”.
To write the monatomic anion a suffix (-ide) has to be added after the element name. Halogen Chlorine forms anion with one atom by taking one electron from donor. To write this monatomic ion (Cl-) we use the name “Chloride”.
Monatomic cation with various oxidation states
For the cationic elements which shows more than one oxidation states means they can be energetically stable in different cationic configuration, are written by their atomic symbol with that oxidation state in Roman numeral in a bracket.
Mainly transitional metals form this type of monatomic ion examples with more than one oxidation states. For the presence of the “d” orbital in these they can form different cationic configuration. For example Iron can be stable in both forms Fe (l) and Fe (ll).
Conclusion
By warping up this article it can be concluded that for gaining energetic stability with full filled orbital, elements form these ions which are monatomic ion examples.

Hi…I am Triyasha Mondal, pursuing M.Sc in Chemistry. I am an enthusiastic learner. My specialization is in physical chemistry.
Let’s connect through LinkedIn: