The electronic configuration of an element is used to describe the arrangement of its electrons in its atomic orbitals. Let us discuss the energy levels that enclose an atom.
The electronic configuration of [I] is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 5p5, with atomic number 53. Iodine (I) is located in the p-block of the periodic table. It belongs to period 5 and group 17.
This article will discuss information on the electronic configuration of iodine, such as the writing of its electronic configuration and the orbital diagram of ground-state Iodine.
How to write the Iodine electronic configuration
The electronic configuration of [I] is derived using the following steps illustrated.
- Iodine is a 5-period element with a total of 53 electrons, filled in the ascending series of energy levels 1s, 2s, 2p, and so on.
- The s, p, d and f orbitals can hold a maximum of 2, 6, 10 and 14 electrons respectively.
- The configuration of I is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 after filling 48 electrons entirely with respective subshells.
- The remaining 5 electrons are filled in the available subshell p, according to Hund’s rule, 3 electrons will be filled, leaving 2 electrons. Then the last subshell will be left with two electrons- that is 5p5.
- Thus the complete electronic configuration of [I] is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 5p5.
Iodine electron configuration diagram
The electronic configuration of [I] is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 5p5. The diagram drawn is as follows, where-
- The s orbital can hold a maximum of 2 electrons
- The p orbital can hold a maximum of 6 electrons
- The d orbital can hold a maximum of 10 electrons and
- The f orbital can hold a maximum of 14 electrons.

Iodine electron configuration notation.
- The electronic configuration notation of [I] is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 5p5.
- The electronic configuration notation of I is also written as [Kr] 4d10, 5s2, and 5p5, where 36 electrons come from the Krypton noble gas configuration, while the remaining 17 are distributed in the notation.
Iodine unabbreviated electron configuration
The unabbreviated electronic configuration of I is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5.
Ground state Iodine electron configuration
The ground state electrical configuration is I is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5.
Excited state Iodine electron configuration
- The first excited state electronic configuration of [I] is [Kr] 4d10, 5s2, 5p4, and 5d1, which is characterized by the transition of one electron from 5d to 6s orbital, creating 5d8 and 6s2 in the outermost shell.

- The second exciting state electronic configuration of iodine is 4d10, 5s2, 5p3, 5d2, resulting from jumping of another molecule from 5p to 5d.
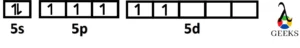
Ground state Iodine orbital diagram
The ground state electronic configuration of I is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5 and the orbital diagram is drawn using the following steps.
- At first, the orbitals are arranged in increasing order of energy.
- Then the electrons will be filled according to Aufbau’s principle, Hund’s rule, and Paulis exclusion principle.
- Thus, the ground state orbital diagram represents the electronic configuration of I as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5.

Conclusion
An atom’s electrical configuration is expressed as the sum of the energies of the orbitals in which its electrons are located. Iodine has 53 electrons in its outermost shell, with electronic configuration notation [Kr] 4d10 5s2, 5p5. It has two excited states involving the transition of 1 electron from the 5p to 5d spin configuration.
Also Read:
- Aluminium electron configuration
- Helium electron configuration
- Europium electron configuration
- Curium electron configuration
- Nobelium electron configuration
- Fluorine electron configuration
- Sulfur electron configuration
- Lawrencium electron configuration
- Thulium electron configuration
- Praseodymium electron configuration
A polymer scientist, teacher, and consultant, Dr. Deepak Poddar is the Guest Faculty in the Department of Chemistry at the Netaji Subhas University of Technology, Delhi. An alumnus of the University of Delhi (B.Sc.) and CIPET, Ahmedabad (M.Sc.), He received Ph.D. in Chemistry (specializing in Biomaterials) from the University of Delhi, India, under the guidance
of Professor Purnima Jain. His research area spans biomaterials, polymer
functionalization, nanomaterials, and tissue engineering. He emphasizes a multi-disciplinary approach to solving problems and believes in solid collaborative efforts