This article discusses about ideal gas properties. The term ideal means something that is perfect in all aspects. Although nothing in this world is ideal.
Be it humans, animals or any other part of nature, nothing is ideal. This is the way of life, with bad only we can see good. Let us limit our discussion to gases. In actual cases there are only real gases that exist. There are some gases which are nearly ideal. In this article we shall study about properties of an ideal gas and then study further about the real gases that are nearly ideal.
- Ideal gas properties of air
- Ideal gas properties of Methane
- Ideal gas properties of Co2
- Ideal gas properties of Helium
- Ideal gas properties of Nitrogen
- Ideal gas properties of Argon
- Ideal gas properties of Propane
- Ideal gas properties of Oxygen
What is an ideal gas?
An ideal gas is defined as the theoretical gas which has no inter particle interactions but consists of many point particles moving randomly or following Brownian motion.
In reality the ideal gases do not exist and only real gases exist. Ideal gases follow some of the laws that are considered as basic characteristics of an ideal gas. The real gases which nearly follow these laws or characteristics are also considered as ideal gases. Let us study in the secton below about the characteristics of an ideal gas.
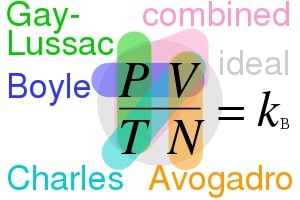
Image credits: Cmglee, Ideal gas law relationships, CC BY-SA 4.0
Characteristics of an ideal gas
The characteristics of ideal gas is given by the kinetic theory of gases. These characteristics are given in the section below-
- The molecules of gas follow a brownian motion and move constantly.
- The path travelled by the molecules of gas follow a straight line and it does not change unless it collides with other molecule or container.
- There is no interaction between the molecules that is there is no attractive force or replusive force acting between the molecules.
- The gas particles are considered to be very small or point masses. They do not hold any amount of volume.
- The collisions between these point masses are elastic. Energy is conserved in this entire process that is there is no gain or loss of energy in this process.
- The molecules at the same temperature have same kinetic energy.
Types of Ideal gas
The ideal gases are classfied into three major types. These types are given in the section below-
- Classical or Maxwell-Boltzmann ideal gas – This type of gas is further divided into classical thermodynamic ideal gas and ideal quantum Boltzmann gas.
- Ideal quantum Bose gas– This type of gas is governed by the Bose-Einstein statistics and the distribution of energy of these Bosons will be in the form of Bose-Einstein distribution.
- Ideal quantum Fermi gas– This type of gas is governed by Fermi-Dirac statistics and the distribution of energy of these Fermions will be in the form of Fermi-Dirac distribution.
Ideal gas properties of air
The table below shows the ideal gas properties of air. Air is a mixture of many gases but it follows some characteristics of an ideal gas.
A gas behaves as an ideal gas when the temprature is high and pressure is low. Air under such conditions behaves like an ideal gas.
The table below shows the properties of air
Molar mass- 28.97
Gas constant- 0.287
Cp- 1.005
Cv- 0.718
Ideal gas properties of Methane
Methane is a carbon compound made up of single carbon atom and four hydrogen atoms bonded to it. The properties of Methane is given in the section given below
A gas behaves as an ideal gas when the temperature is high and pressure is low. Methane under such conditions behaves like an ideal gas.
Density- 0.657 kg/m3
Melting Point- -183 degrees celsius
Boiling Point- -162 degrees celsius
Critical temperature- 190.56 K
Ideal gas properties of Co2
Co2 or carboon dioxide is also a carbon compound made up of single carbon atom and two oxygen atoms bonded to it. Csrbon dioxide is exhaled by us humans. Co2 is also used as fire extinguisher as it does not support combustion.
A gas behaves as an ideal gas when the temperature is high and pressure is low. Co2 under such conditions behaves like an ideal gas.
The ideal gas properties of CO2 are given in the section given below-
Molar mass- 44.01
Gas constant- 0.1889
Cp – 0.846
Cv- 0.657
Ideal gas properties of Helium
Helium is a noble gas and has an atomic number of 2. It has only one shell which can fit two electrons in it. The Helium atom is stable as the shell is full and is at the lowest possible energy state.
A gas behaves as an ideal gas when the temperature is high and pressure is low. Helium under such conditions behaves like an ideal gas.
The ideal gas properties of Helium are given in the section below-
Molar mass – 4
Gas constant- 2.07
Cp- 5.9
Cv- 3.11
Ideal gas properties of Nitrogen
Nitrogen gas is a colourless chemical element. It does not have any odour and is inert and non metallic in nature. The ideal gas properties of Nitrogen are given in the table given below.
A gas behaves as an ideal gas when the temperature is high and pressure is low. Nitrogen under such conditions behaves like an ideal gas.
Molar mass- 28
Gas constant- 0.29
Cp- 1.039
Cv- 0.743
Ideal gas properties of Argon
Like Nitrogen, Argon is also a colourless and odourless chemical element. It is non flammable in nature and involatile. It belongs to the Noble gas family whose outermost shell is completely filled which makes the atom more stable.
A gas behaves as an ideal gas when the temperature is high and pressure is low. Argon under such conditions behaves like an ideal gas.
The ideal gas properties of Argon are given in the section below-
Molar mass- 40
Gas constant- 0.2081
Cp- 0.52
Cv- 0.31
Ideal gas properties of Oxygen
Oxygen is a gaseous chemical element which is also odourless and colourless. In addition to that we can say that Oxygen supports combustion, as it acts as an oxidizer and combustion needs an oxidizing agent to take place. We all inhale oxygen to survive.
A gas behaves as an ideal gas when the temperature is high and pressure is low. Oxygen under such conditions behaves like an ideal gas.
The ideal gas properties of Oxygen are given in the section given below–
Molar mass- 32
Gas constant- 0.25
Cp- 0.918
Cv- 0.65
Ideal gas properties of Propane
Propane belongs to the alkane family. Propane is a carbon compound having three carbon atoms and eighth hydrogen atoms bonded to it. It is a very unique element as Propane is environment friendly.
A gas behaves as an ideal gas when the temperature is high and pressure is low. Propane under such conditions behaves like an ideal gas.
The ideal gas properties of Propane gas are given in the section below–
Molar mass- 44
Gas constant- 0.1885
Cp- 1.67
Cv- 1.49
Pressure temperature relationship of an ideal gas
The pressure and temperature for an ideal gas are related to each other by the equation given in the section below-
PV = nRT
Where,
P is the pressure of the ideal gas
V is the volume of the gas
n is the number of moles of gas
R is the universal gas constant
T is the temperature of the gas in the system
Hi ….I am Abhishek Khambhata, have pursued B. Tech in Mechanical Engineering. Throughout four years of my engineering, I have designed and flown unmanned aerial vehicles. My forte is fluid mechanics and thermal engineering. My fourth-year project was based on the performance enhancement of unmanned aerial vehicles using solar technology. I would like to connect with like-minded people.