In the realm of physics, understanding the principles of heat exchange is crucial for a wide range of applications, from power generation to refrigeration and cooking. This comprehensive guide will delve into the intricacies of finding energy transfer in heat exchange, providing you with the necessary tools and knowledge to tackle this fundamental concept.
The Heat Transfer Equation: Unlocking the Secrets of Energy Transfer
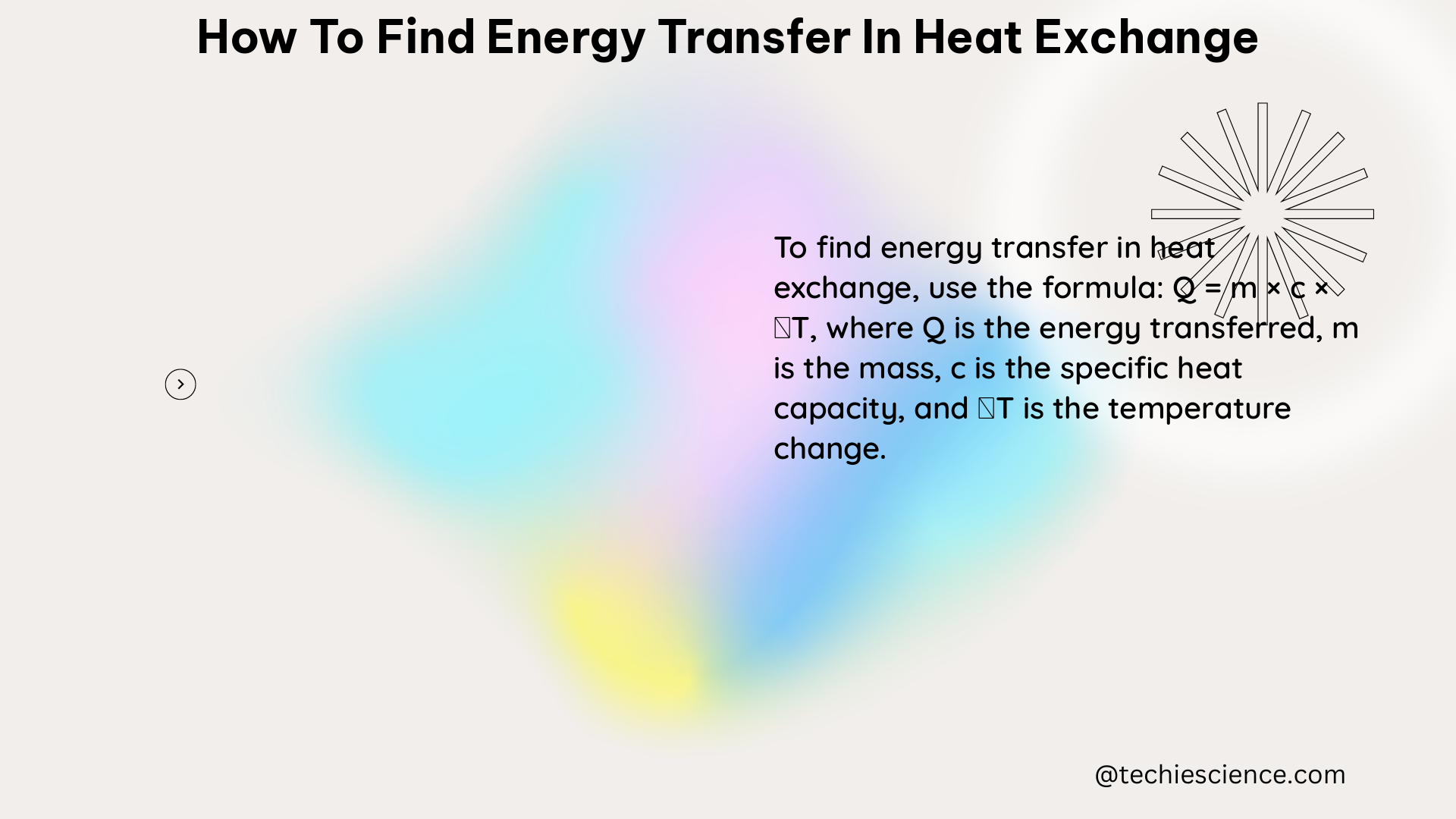
At the heart of heat exchange lies the heat transfer equation, which serves as the foundation for quantifying the amount of energy transferred. The equation is expressed as:
[
q = mc \Delta T
]
where:
– q is the amount of heat transferred (in joules, J)
– m is the mass of the substance (in kilograms, kg)
– c is the specific heat capacity of the substance (in joules per kilogram per degree Celsius, J/kg°C)
– ΔT is the change in temperature (in degrees Celsius, °C)
This equation allows us to calculate the energy transfer in various heat exchange scenarios, providing a powerful tool for understanding and predicting the behavior of thermal systems.
Specific Heat Capacity: The Key to Unlocking Energy Transfer
The specific heat capacity of a substance is a crucial parameter in the heat transfer equation. It represents the amount of heat required to raise the temperature of one kilogram of a substance by one degree Celsius. This value varies for different materials, and understanding these variations is essential for accurate energy transfer calculations.
Here are some examples of specific heat capacities for common substances:
– Water: c = 4.184 J/kg°C
– Copper: c = 0.385 J/kg°C
– Aluminum: c = 0.897 J/kg°C
Knowing the specific heat capacity of the materials involved in a heat exchange process is crucial for determining the energy transfer accurately.
Calorimetry: Measuring Heat Transfer in the Lab
Calorimetry is a powerful experimental technique used to measure the heat transfer in chemical and physical processes. It involves using a calibrated substance, typically water, to determine the amount of heat absorbed or released during a reaction or phase change.
The process of calorimetry involves the following steps:
1. Placing the substance of interest in a calorimeter, which is a well-insulated container designed to minimize heat loss or gain from the surroundings.
2. Measuring the initial temperature of the calibrated substance (usually water) in the calorimeter.
3. Introducing the substance of interest into the calorimeter and allowing the system to reach thermal equilibrium.
4. Measuring the final temperature of the calibrated substance.
5. Calculating the heat transfer using the heat transfer equation.
By employing calorimetry, researchers and students can directly measure the energy transfer in various heat exchange processes, providing valuable insights and data for further analysis.
Examples and Practice Problems: Applying the Concepts
To solidify your understanding of energy transfer in heat exchange, let’s explore some practical examples and practice problems.
Example 1: Calculating Heat Transfer for Water
Suppose you want to calculate the heat transfer required to raise the temperature of 1 kg of water from 20°C to 80°C. Using the heat transfer equation, we can solve this problem:
[
q = mc \Delta T = 1 \text{ kg} \times 4.184 \text{ J/kg°C} \times (80°C – 20°C) = 251.2 \text{ kJ}
]
Example 2: Calculating Heat Transfer for Copper
Now, let’s consider the heat transfer required to raise the temperature of 0.5 kg of copper from 20°C to 50°C. Applying the heat transfer equation, we get:
[
q = mc \Delta T = 0.5 \text{ kg} \times 0.385 \text{ J/kg°C} \times (50°C – 20°C) = 9.625 \text{ kJ}
]
These examples demonstrate how to use the heat transfer equation and the specific heat capacity values to calculate the energy transfer in different heat exchange scenarios.
Real-World Applications: Harnessing Heat Transfer
Heat transfer is a fundamental concept that underpins a wide range of real-world applications. Understanding energy transfer in heat exchange is crucial in the following areas:
-
Power Plants: In power plants, heat energy is converted into mechanical energy to generate electricity. The efficiency of this process relies on the effective transfer of heat between various components, such as boilers, turbines, and condensers.
-
Refrigeration: Refrigeration systems work by transferring heat from a colder body (the refrigerant) to a hotter body (the surrounding environment). This process is essential for cooling and freezing applications, such as in household refrigerators and air conditioning units.
-
Cooking: Cooking involves the transfer of heat from a heat source (e.g., stove, oven) to the food. Understanding the principles of heat transfer helps chefs and home cooks optimize their cooking techniques and achieve desired results.
-
Industrial Processes: Heat exchange is crucial in various industrial processes, such as chemical reactions, distillation, and heat treatment of materials. Accurate understanding of energy transfer helps optimize these processes for efficiency and productivity.
-
Building Design: The design of buildings, including their insulation, ventilation, and heating/cooling systems, relies on the principles of heat transfer to maintain comfortable indoor environments and minimize energy consumption.
By mastering the concepts of energy transfer in heat exchange, you can unlock a deeper understanding of these real-world applications and contribute to advancements in various fields, from energy production to product design and beyond.
Conclusion
In this comprehensive guide, we have explored the fundamental principles of finding energy transfer in heat exchange. From the heat transfer equation to the importance of specific heat capacity and the role of calorimetry, you now have a solid foundation to tackle this crucial concept in physics.
By applying the knowledge and techniques presented here, you can confidently analyze and solve a wide range of heat exchange problems, both in the classroom and in real-world scenarios. Remember to practice regularly, explore additional resources, and continuously expand your understanding of this fascinating and versatile topic.
References
- Lumen Learning. (n.d.). Temperature Change and Heat Capacity. Retrieved from https://courses.lumenlearning.com/suny-physics/chapter/14-2-temperature-change-and-heat-capacity/
- Fiveable. (2024). Heat Capacity and Calorimetry. Retrieved from https://library.fiveable.me/ap-chem/unit-6/heat-capacity-calorimetry/study-guide/jShImkrhZMnPWxlEjdwN
- LibreTexts. (2022). Calorimetry. Retrieved from https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_1e_%28OpenSTAX%29/05:_Thermochemistry/5.2:_Calorimetry
- Arami, A. C. (1999). Measurements of Heat Transfer in Physical and Chemical Processes. Retrieved from https://uh.edu/honors/Programs-Minors/honors-and-the-schools/houston-teachers-institute/curriculum-units/pdfs/1999/technology-and-the-discipline-of-chemistry/arami-99-chemistry.pdf
- LibreTexts. (2022). Quantifying Heat and Work. Retrieved from https://chem.libretexts.org/Courses/College_of_the_Canyons/Chem_201:_General_Chemistry_I_OER/08:_Thermochemistry/8.03:_Quantifying_Heat_and_Work

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.