In a chemical reaction chemical symbols are used to representing reactant and product. Let us discuss about the reaction between HF and SO3 .
Hydrogen fluoride or HF is a gas but when dissolved in water forms the strongest hydro flouric acid. Sulphur trioxide or SO3 is also called Nisso Sulphan which is a white crystalline solid substance. HF has a unpleasant smell with 20.006 g/mol molar mass.
HF is mostly used for production of fluorine, pharmaceutical and polymers. Let us discuss about HF+ SO3 with all details in the following sections.
What is the product of HF and SO3
HF reacts with SO3 to form fluoro sulphonic acid.
HF + SO3 ——–> HFSO3
What type of reaction is HF + SO3
HF + SO3 is a combination reaction. In this reaction both the reactants HF and SO3 combines together to form only one product HFSO3.
How to balance HF + SO3
The unbalanced equation of HF + SO3 is
HF + SO3 ——> HFSO3
- Assigning some coefficients to both reactants and products
- a HF + b SO3 ——-> c HFSO3
- An equation is made with the above equation
- H= a= c, F=a =c, S =b = c, O=3b = 3c
- The linear equation made is a=c,b=c, 3b =3c
- Solve the above equation using gauss elimination method. The values of coefficients is
- The values of coefficients assigned is a =1, b= 1, c= 1
- The balanced equation of HF + SO3 is
- HF + SO3 ——> HFSO3
HF + SO3 titration
HF + SO3 titration cant be performed. Because both HF and SO3 are acids. We cant titrate acids together to get the end point.
HF + SO3 net ionic equation
The net ionic equation of HF and SO3
HF + SO3 ——-> HFSO3
- Both HF and SO3 does not dissociate to give the corresponding ions.
HF + SO3 conjugate Pairs
HF + SO3 has the following conjugate pairs,
- The conjugate base of HF is F– or fluoride.
- The conjugate acid of HF is fluoronium. There is no conjugate acid observed for SO3.
HF + SO3 Intermolecular forces
The intermolecular forces exist in between HF + SO3 is
- Dipole dipole interaction is observed in Hydrogen fluoride and Sulphur trioxide.
- In HF there is dipole dipole interaction can be seen in between H+ ion and F– .
- In SO3 electrostatic force of attraction observed between the ions.
HF + SO3 Reaction enthalpy
The reaction enthalpy of HF + SO3 is 342.43 KJ/mol. The enthalpy of formation of HF, HFSO3 and SO3 is -14.99, -395.7 and -753.12 kJ/mol respectively. Hence enthalpy is {-14.99 + -395.7-(-753.12)}. The reaction is found to be endothermic because the reaction enthalpy value is positive.
Is HF + SO3 a buffer solution
HF + SO3 is not a buffer solution. A buffer solution is usually prepared by mixing a weak acid with its conjugate base or vice versa. Here both the reactants are acids. So their mixing will not give a buffer solution.
Is HF + SO3 a complete reaction
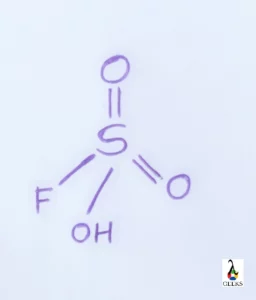
HF + SO3 is a complete reaction. The reaction takes place to form HFSO3, fluoro sulphonic acid a stable product. Fluoro sulphonic acid is a colorless or sometimes an yellow liquid which has a tetrahedral shape. Its melting point is low compared to the boiling point.
Is HF + SO3 an exothermic or endothermic reaction
HF + SO3 is an endothermic reaction. The reaction doesn’t liberate any amount of heat.
Is HF + SO3 a redox reaction
HF + SO3 is not a redox reaction. There will be a change in oxidation number of reactants and products in redox reaction. Here both the oxidation state of all atoms in the reactant side and product side is same.
Is HF + SO3 a precipitation reaction
HF + SO3 is reaction doesn’t form any precipitate. It is because the product formed strontium iodide is soluble in water will get dissolved and not precipitated.
Is HF + SO3 reversible or irreversible reaction
HF + SO3 is an irreversible reaction. The reaction can’t be reversed to yield back its reactants
Is HF + SO3 a displacement reaction
HF + SO3 is a combination reaction where both the reactants undergo combination to form the products.
Conclusion
Hydrogen fluoride is a linear shape molecule which is corrosive and sometimes irritant. SO3 is used as a precursor for making sulphuric acid having 80.066 g/mol molar mass.

Hi… I am Aparna Dev, a chemistry Postgraduate with a good understanding of chemistry concepts. I am working in Kerala Minerals and Metals Limited Kollam with experience in the development of electrocatalysts as a part of post graduate thesis.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aparna-dev-76a8751b9