HCN Lewis dot structure is of great significance in terms of understanding the number of bond pairs, lone pairs, and type of bonds involved. Though the structure seems simple many underlying complexities to are going to be discussed in this article.
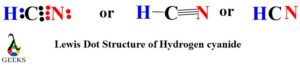
HCN Lewis dot structure consist of 3 elements as shown in the formula. Due to electronegativity difference carbon is the central atom which shares its 1 electron with hydrogen and 3 electrons with nitrogen to fulfill the stability criteria. This leads to formation of carbon forming single covalent bond with hydrogen and triple covalent bond with nitrogen.

Elaborating more on HCN Lewis dot structure then it comprises 3 elements namely hydrogen, carbon, and nitrogen. To determine the HCN Lewis Dot Structure first we need to look for valence electrons in individual atoms. Hydrogen (Atomic number = 1 and electronic configuration = 1) belongs to the 1st group of the periodic table and consists of only 1 electron. Similarly carbon (Atomic number = 6 and electronic configuration = 2,4) and nitrogen (Atomic number = 7 and electronic configuration = 2,5) belong to group 14 and 15 and consist of 4 and 5 valence electrons respectively.
It is also important to look out for the central atom when determining the HCN Lewis Dot Structure . The central atom can be identified by determining the electronegativity of all the elements present in the compound. The electronegativity value of C, N, and O are 2.5, 3.5, and 2.1. Usually, the atom with the least electronegativity is considered for the position of the central atom. Here hydrogen is the least electronegative but it cannot occupy the central position because of the large electronegativity difference between hydrogen and carbon. Hence vectorially the movement of charge will be from hydrogen to carbon. So carbon will take the central position and nitrogen and hydrogen will be terminal atoms.
To complete its stability requirement hydrogen will share its 1 electron with 1 electron of carbon thereby forming a single (C-H) covalent bond. Carbon will share its remaining 3 electrons with nitrogen to achieve octet stability for itself and nitrogen. Due to sharing of 3 electrons between carbon and nitrogen, a triple covalent bond will be formed. Also, nitrogen will be left with single lone pair of electrons giving the best possible diagrammatic view of hydrogen cyanide.
Hydrogen cyanide or HCN is a chemical compound that is a colorless, extremely toxic, volatile, and flammable liquid with a bitter almond-like smell that usually goes undetectable. It is also called prussic acid when dissolved in water. It is usually said that it’s named prussic acid because it was derived from Prussian blue by Swedish chemist Carl Wilhelm Schlee. Many historical theories and researches believe that hydrogen cyanide was one of the first molecules found on early earth.
Many historical theories and researches believe that hydrogen cyanide was one of the first molecules found on early earth. Many old tales believe that comets and asteroids were responsible for their existence on earth. The chapter 15 of the book Hazardous gases published in 2021 and titled ‘Hydrogen cyanide: Risk assessment, environmental, and health hazard’ authors Manila and Payal Devi mentioned that current research has proven experimentally that during the early earth time hydrogen cyanide used to be a prerequisite or basic feedstock molecule responsible for the formation of sugars, nucleotides, and various other biomolecules. These biomolecules were the basis of the origin of life on earth thereby representing a significant contribution of HCN in the origin of life.
Elaborating on its structural properties, then HCN is a linear molecule and is of sp hybridization. Its bond angle is 180 degrees and it boils at room temperature only. In terms of solubility, it is completely miscible in water and ethanol. Another important property shown by it is that of tautomerism. HCN also has a tautomer named hydrogen isocyanide (HNC). The concept of tautomerism is of great importance in organic chemistry. Another concept explained by HCN and HNC is ambident nucleophiles which are again commonly discussed in organic synthesis.

The occurrence of HCN and the research conducted on it is quite vast and overwhelming. It is quite informative. Despite being highly poisonous it is found in a variety of unexpected places. In terms of natural occurrence, it is found in fruits with pits like apples, bitter almonds, cherries, etc. It is said that these pits contain cyanohydrins pigment which releases HCN. Many mass spectrometric analyses have shown the presence of HCN in Titan’s atmosphere.
In human physiology, HCN production has a very crucial role to play in neurotransmission, phagocytosis, and vasodilation. Many extensive and in-depth studies have detected the presence of HCN in the interstellar medium. Its formation and destruction have been noticed by high-level telescopes where it is found in the environment of stars.
HCN presence is not only famous in stories of space and the origin of life but it does have an instrumental role to play in the industry as well. It is the precursor to some well-known industrial compounds like sodium cyanide (NaCN) and potassium cyanide (KCN) which are major players in the gold and silver mining and electroplating industry.
. In the polymer industry, it has acted as an intermediate in the synthesis of monomer methyl methacrylate and Nylon 6,6. In the pesticide industry, it is used as a fumigant globally to protect food production facilities. It has proven as a good fumigant in terms of efficacy, application, and minimal environmental harm.
Ironically HCN presence is ubiquitous but it is still treated as one of the most dangerous and toxic compounds to be used. Unfolding pages of history are the evidence that it was used as one of the most dreaded chemical weapons. Direct human exposure to HCN through the air, water, food, touching cyanide soil, or through smoking is really bad for health. This is because it prevents the human cells from using oxygen and in turn affects the functioning of the heart and brain. So it is really important to protect ourselves from it. Many countries have issued specific guidelines on cyanide usage because even after surviving it causes uncontrollable damage.
HCN Lewis Dot Structure (Related FAQs)
Describe the polarity of HCN Lewis Dot Structure and its affects on polarity
HCN is a polar molecule due to the large electronegativity difference between its terminal atoms nitrogen and hydrogen. This electronegativity difference leads to a partial positive charge on the hydrogen end and a partial negative charge on the nitrogen end. Due to the difference in these charges, a dipole moment also exists. This dipole moment is responsible for the solubility of HCN in water.
Why HCN is considered as a weak acid ?
HCN is considered a weak acid as compared to HCl or H2SO4 because when dissolved in water or an aqueous medium it partially dissociates into hydronium ion and cyanide ion. On the other hand, hydrochloric acid and sulphuric acid completely dissociate in an aqueous medium. The factors for its low dissociation are the less electronegativity difference and less polarity as compared to strong acids
Define tautomerism as represented by HCN Lewis Dot Structure
Tautomerism is a kind of structural isomerism where there are no strict barriers in terms of interconversion of isomers. The structures are called tautomers and exist in dynamic equilibrium.
Explain about ambident nucleophilicity of HCN Lewis Dot Structure
Yes, HCN is an ambident nucleophile. It can be defined as an anionic nucleophile with two nucleophilic centers or two negative sites. Over here negative charge delocalization is also observed due to resonance. HCN and HNC are good examples of this concept which is used in organic synthesis.

Hello, I am Mansi Sharma, I have completed my master’s in Chemistry. I personally believe that learning is more enthusiastic when learnt with creativity. I am a Subject Matter Expert in Chemistry.
Let’s connect through LinkedIn: https://www.linkedin.com/in/mansi-sharma22