Li3N is a strong lewis base that can easily react with a strong acid like HCl. Let us see what happens when both species are reacting together and know the fact.
After ammonia, lithium nitride is one of most strong lewis bases where all the lone pairs over nitrogen are available for donation. It can easily react with a strong acid like hydrochloric acid followed by an acid-base reaction also the lone pair over Li3N can be donated and it was accepted in the vacant orbital of Cl.
The reaction will not require any kind of catalyst, or temperature because both species are more reactive. In this article, we will learn the mechanism of the reaction like enthalpy, redox, precipitation, product formation, type of reaction, etc in detail.
1. What is the product of HCl and Li3N?
When HCl reacts with Li3N by 3:1 proportion then LiCl and NH3 form but when excess HCl is reacted with by 4:1 proportion then ammonium chloride is formed along with LiCl.
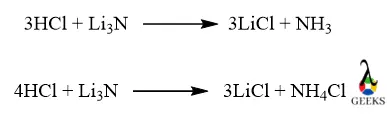
Between HCl and Li3N
2. What type of reaction is HCl + Li3N?
The reaction between HCl + Li3N is an acid-base reaction along with a displacement reaction where Cl gets displaced, it is also an example of a redox reaction along with a donor-acceptor reaction.
3. How to balance HCl + Li3N?
HCl + Li3N = LiCl + NH3 and HCl + Li3N = LiCl + NH4Cl both reactions are not balanced yet, because in both reactions left-hand side is not equal to the right-hand side. Now we have to balance both equations in the following steps-
- Step 1 – Label all the molecules in the reactant sites as well as the product site.
- All the molecules are labeled by the English alphabet like A, B, C, and D because four molecules are present in both reactions. Then both reactions look like this,
- A HCl + B Li3N = C LiCl + D NH3
- E HCl + F Li3N = G LiCl + H NH4Cl
- Step 2 – Equating all the alphabet and coefficients of the molecules making a particular equation,
- H = A = 3D, Cl = A = C, Li = 3B = C, N = B = D and for other reactions the equations will be, H = E = 4H, Cl = E = G = H, Li = 3F = G, N = F = H
- Step 3 – Now using Gaussian elimination we calculate the value of each coefficient and variable,
- By using Gaussian elimination and substitution we can solve both equations and get, A = 3, B = 1, C = 3, D = 1 and E = 4, F = 1, G = 3, and H = 1.
- So, overall balanced equations are,
- 3 HCl + Li3N = 3 LiCl + NH3
- 4 HCl + Li3N = 3 LiCl + NH4Cl
4. HCl + Li3N titration
Li3N can be titrated against HCl which is an acid-base titration with a proper indicator to estimate the strength of the acid as well base. The titration required a few more things which are listed below,
Apparatus used
We need a burette, conical flask, burette holder, volumetric flask, and beakers.
Titre and titrant
Here HCl is served as titrant which is taken in the burette and the molecule to be analyzed is Li3N as the titer.
Indicator
As it is an acid-base titration so the best-fit indicator is here phenolphthalein because in the basic medium it shows a pink color and acidic medium it becomes colorless, so we easily get the endpoint.
Procedure
The burette is filled with HCl and Li3N is taken in a conical flask and added a few drops of indicator and shaken well. No start the titration by adding HCl from the burette dropwise in the conical and constant shaking.
After a certain time, the indicator changes its color which indicates that the base is fully neutralized by the acid and stops the titration. We repeat the titration several times for better results and then we find out the strength of the acid by the formula V1S1 = V2S2.
5. HCl+ Li3N net ionic equation
Several ions are formed in the reaction between HCl + Li3N like proton and chloride and Li+, N3- NH3 could not be ionized but NH4Cl can be ionized like NH4+ and Cl–.
- 3H+ + 3Cl– + 3Li+ + N3- = 3Li+ + 3Cl– + NH3
- 4H+ + 4Cl– + 3Li+ + N3- = 3Li+ + 3Cl– + NH4+ + Cl–
6. HCl+ Li3N conjugate pairs
In the reaction HCl+ Li3N the conjugate pair of HCl is the corresponding base Cl– and the conjugate acid of NH3 is the corresponding acid NH4+ which can be formed in the course of the reaction.
- Conjugate pair of HCl = Cl–
- Conjugate pair of NH3 = NH4+
7. HCl and Li3N intermolecular forces
Co-valent and dipole interaction present in HCl and ionic interaction present in Li3N and also strong electrostatic force due to the high charge density of both elements, and strong ionic force present for NH4Cl and LiCl as both are electrolytes and also van der waal’s force present.
| Molecule | Acting force |
| 1.HCl | Electrostatic, van der waals, Dipole interaction |
| 2.Li3N | Ionic, electrostatic |
| 3.LiCl | Strongly Ionic, van der waal’s |
| 4.NH4Cl | electrostatic force, covalent |
| 5.NH3 | H-bonding, covalent |
8. HCl + Li3N reaction enthalpy
The enthalpy change, for the reaction 3 HCl + Li3N = 3 LiCl + NH3 is -828.77 KJ/mol whereas for the reaction 4 HCl + Li3N = 3 LiCl + NH4Cl is -514.47 KJ/mol. We have to calculate the reaction enthalpy separately for two reactions of the different stoichiometric proportions of HCl.
| Molecule | Enthalpy (KJ/mol) |
| 1.Li3N | -165.14 |
| 2.HCl | -92.3 |
| 3.LiCl | -408.27 |
| 4.NH3 | -46 |
| 5.NH4Cl | +176 |
9. Is HCl + Li3N a buffer solution?
The reaction between HCl + Li3N gives a buffer solution of NH4Cl which can control the pH of the solution if any acid or base is added to this solution.
10. Is HCl + Li3N a complete reaction?
The reaction between HCl + Li3N completely react to form different products like LiCl, NH3, and NH4Cl with different stoichiometric proportions and utilized the reactants.
11. Is HCl + Li3N an exothermic or endothermic reaction?
The reaction between HCl + Li3N both two are exothermic reaction having different stoichiometric reactions due to the negative value of enthalpy in terms of thermodynamics, which release heat in course of the reaction and that heat provide energy for the reactions.
12. Is HCl + Li3N a redox reaction?
The above reaction between HCl + Li3N is a redox reaction where Cl gets reduced and N gets oxidized along with Li, where the oxidation number changes during the course of the reaction.

Between HCl and Li3N
13. Is HCl + Li3N a precipitation reaction
The reaction between HCl + Li3N is a precipitation reaction because NH4Cl gets precipitated when 4 moles of HCl react with Li3N, but when it forms NH3 due to its gaseous nature it cannot be soluble in water but not in precipitated form.
What is the Reaction Between HCl and CaCO3, and How Does it Compare to the Reaction Between HCl and Li3N?
The HCl CaCO3 reaction is an acid-base reaction where calcium carbonate (CaCO3) reacts with hydrochloric acid (HCl), producing carbon dioxide (CO2), water (H2O), and calcium chloride (CaCl2). On the other hand, the HCl Li3N reaction is a neutralization reaction where lithium nitride (Li3N) reacts with HCl to form lithium chloride (LiCl) and ammonia gas (NH3). Both reactions involve the combination of HCl with different compounds, resulting in distinct products. Hcl caco3 reaction elements explained.
14. Is HCl + Li3N reversible or irreversible reaction?
The reaction between 4 moles of HCl + Li3N is reversible in nature the reaction can proceed in both forward and backward directions but the reaction between 3 moles of HCl + Li3N is irreversible causing NH3 cannot reacts further.
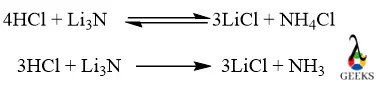
Reactions of HCl and Li3N
15. Is HCl + Li3N displacement reaction?
Both reactions of Li3N + HCl of higher or lower moles are displacement reactions because in both cases Li is displaced and attached to the Cl part and N is also displaced and attached to H.
Conclusion
The reaction between HCl and Li3N can occur in different moles of HCl and get a different product, where higher moles of HCl gives NH4Cl and lower moles give NH3 along with NH3. So, we can use this reaction for the production of NH4Cl and NH3 as well, and the reaction is can be used for the estimation of Chloride as well as nitride ions.
Read more facts on HCl:

Hi……I am Biswarup Chandra Dey, I have completed my Master’s in Chemistry from the Central University of Punjab. My area of specialization is Inorganic Chemistry. Chemistry is not all about reading line by line and memorizing, it is a concept to understand in an easy way and here I am sharing with you the concept about chemistry which I learn because knowledge is worth to share it.