Hydrochloric acid and Hypochlorous acid are both inorganic acids. Let us inspect some interesting facts about the reaction between HCl and HClO.
Hydrochloric acid is a stronger acid than Hypochlorous acid. Hypochlorous acid serves as a base in the reaction medium. Concentrated hydrochloric acid is needed in this reaction. This reaction readily proceeds on cold water-ice and nitric acid trihydrate (NAT) surfaces.
Let us thrive into the mechanism of the reaction between HCl and HClO with some FAQs with proper explanation in the following article.
1. What is the product of HCl and HClO?
Water is produced along with the evolution of chlorine gas when hydrochloric acid and hypochlorous acid react.
HCl (aq) + HClO (aq) -> H2O (l) + Cl2(g)
2. What type of reaction is HCl + HClO?
The reaction between hydrochloric acid and hypochlorous acid is an example of a double displacement reaction. It also falls under the category of redox and gas evolution reactions. In hydrochloric acid, chlorine gets oxidized; in hypochlorous acid, chlorine is reduced to give diatomic chlorine gas.
3. How to balance HCl + HClO?
To balance the equation between HCl and HClO, we have to check if an equal number of individual atoms (H, Cl, O) are present on both the reactant and product sides.
- Step 1- We first label each molecule as A, B, C, and D, as four elements are present.
- The reaction looks like this,
- A HCl + B HClO = C H2O + D Cl2
- Step 2- Now we calculate the number of coefficients labelled as alphabets in reactants and products using suitable numbers.
- H = A = B= 2C, Cl = A = B = 2D, O= B = C
- Step 3- We calculate the coefficient and variables required to balance the equation using Gaussian elimination process. Now we get,
- A = 1, B = 1, C = 1, and D =1
- The reaction was already balanced.
- So, overall balanced equation for the above reaction is,
- HCl + HClO = H2O + Cl2
4. HCl+HClO titration
The titration of HCl with HClO will not yield a significant change of color as it is hard to detect the end-point/equivalence point.
5. HCl + HClO net ionic equation
Hydrochloric acid will be dissociated as proton and chloride ions. Hypochlorous acid will dissipate as hydroxide ion and chlorine (+1) cation.
H+ + Cl– + OH–+ Cl+ = OH– + H+ + Cl– + Cl+
6. HCl + HClO conjugate pairs
The conjugate pairs of the reaction between hydrochloric acid and hypochlorous acid are the conjugate base of the hydrochloric acid and the respective conjugate acid of the hypochlorous acid, which is shown below,
HCl(-1) + HCl(+1)O = H+OH–+ Cl(+1)Cl(-1)
7. HCl and HClO intermolecular forces
On the reactant side, in HCl, there is an ionic force of attraction between the proton and chlorine atom. In HClO, there is dipole-dipole interaction. On the product side, H2O molecule projects hydrogen bonds, dipole-dipole forces, and London dispersion forces. The intermolecular force that exists in Cl2 is the London dispersion force.
| Molecule | Intermolecular forces |
| HCl | Electrostatic, Van der waals |
| HClO | Dipole dipole |
| H2O | Electrostatic, H-bonding, covalent |
| Cl2 | London dispersion, covalent |
8. HCl + HClO reaction enthalpy
The total enthalpy change is -285,835= -285,820 – (92-77)) KJ/mol, which is negative. Energy will be released in this reaction. Mathematically, ΔH = Hproducts-Hreactans .
| Molecule | Enthalpy (of formation) (KJ/mol) |
| HCl | 92 |
| HClO | -77 |
| H2O | -285,820 |
| Cl2 | 0 |
9. Is HCl + HClO a buffer solution?
The solution of HCl and HClO is a buffer of the weak acid HClO and the conjugate base ClO–. When HCl and HClO are mixed, ClO– will consume H+.
10. Is HCl + HClO a complete reaction?
The reaction between hydrochloric acid and hypochlorous acid is not a complete reaction, as the concentration of reactants never reaches zero. Rather the reaction achieves equilibrium after some time.
11. Is HCl + HClO an exothermic or endothermic reaction?
The reaction of HCl + HClO can be inferred as an exothermic reaction as we get a negative enthalpy change for the reaction. Here it is shown in the diagram,
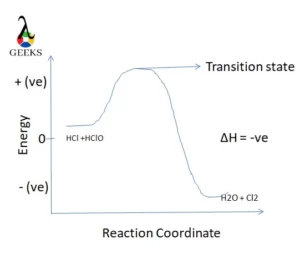
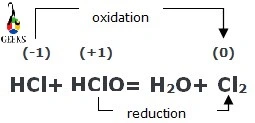
12. Is HCl + HClO a redox reaction?
The reaction between hydrochloric acid and hypochlorous acid is a redox reaction. This redox is a comproportionation reaction type. HClO and HCl act as oxidizing and reducing agents, respectively.
13. Is HCl + HClO a precipitation reaction?
The above reaction is not a precipitation reaction, as we will not get any precipitation as a product.
14. Is HCl + HClO reversible or irreversible reaction?
The above reaction between hydrochloric acid and hypochlorous acid is a reversible reaction. Chlorine reacts reversibly with water, giving the reactants. This property is used for water sterilization.
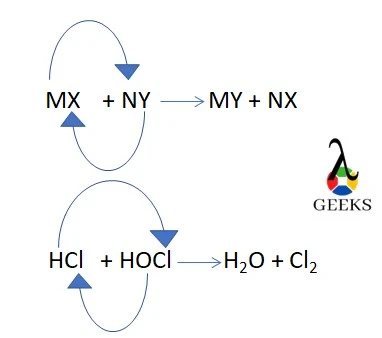
15. Is HCl + HClO displacement reaction?
HCl + HClO is a double-displacement reaction as H+ in HCl displaces Cl– from HClO, and subsequently, OH– in HClO displaces Cl– from HCl, and form H2O and Cl2.
Conclusion
In conclusion Hydrochloric acid and hypochlorous acid form water and chlorine by following a simple inorganic mechanism. This reaction leads to chlorine activation providing atmosphere. Cl2 is a very good oxidizing agent. Thus the reaction has some industrial value.
Read more facts on HCl:

Hey readers, I am Ishita Ghosh. I have done my Master’s in Chemistry. My area of specialization is Inorganic Chemistry. The true way to comprehend chemistry is to understand it from its grassroots level. My effort is to share every bit of knowledge in chemistry I have so that it helps you for a better grasp on this subject.